Grgurić, Toni; Razum, Marta; Martinez, Valentina; Zgrablić, Goran; Senkić, Ana; Karadeniz, Bahar; Etter, Martin; Brekalo, Ivana; Arhangelskis, Mihails; Pavić, Luka et al. | Insights into the conductivity of alkali metal-dhta coordination polymers // Solid-State Science & Research 2025: Book of Abstracts and Programme / Torić, Filip; Ivšić, Trpimir (ur.). | Zagreb: Ruđer Bošković Institute, 2025. str. 73-73
-
Cindro, Nikola; Duvnjak, Mirko; Vidović, Nikolina; Užarević, Krunoslav; Horvat, Gordan; Tomišić, Vladislav; Speranza, Giovanna | Mechanochemical anion-templated synthesis of cyclopeptides // Adriatic NMR conference 2025 : book of abstracts / Bregović, Nikola; Namjesnik, Danijel; Novak, Predrag et al. (ur.). | Croatian Chemical Society, 2025. str. 35-35
adriatic-nmr-conference.hkd.hr
-
Krivačić, Sara; Mendaš, Dora; Martinez, Valentina; Karadeniz, Bahar; Užarević, Krunoslav; Kassal, Petar | Carbonized Metal-Organic Frameworks as Ultra-Hydrophobic Solid Transducer in Potentiometric Potassium Probes // 29th Croatian Meeting of Chemists and Chemical Engineers and 7th Symposium Vladimir Prelog : Book of abstracts / Vrsalović Presečki, Ana (ur.). | Zagreb: Croatian Chemical Society, 2025. str. 68-68
29hskiki.hkd.hr
Vujević, Lucija; Karadeniz, Bahar; Užarević, Krunoslav; Žilić, Dijana; Ilakovac Kveder, Marina | Improving spin coherence of vanadyl-porphyrin qubits in PCN-223 by fullerene encapsulation and dilution of vanadyl ions // Solid-State Science & Research 2025: Book of Abstracts and Programme / Torić, Filip; Ivšić, Trpimir (ur.). | Zagreb: Ruđer Bošković Institute, 2025. str. 33-33
-
Krivačić, Sara; Mendaš, Dora; Martinez, Valentina; Karadeniz, Bahar; Užarević, Krunoslav; Kassal, Petar | Application of metal-organic frameworks as solid-contact in all-solid-state ion-selective electrodes // SUMMER CONFERENCE OF CROATIAN CHEMICAL SOCIETY RIJEKA – PULA 2025 - Book of abstracts / Rezić, Meštrović Iva (ur.). | Rijeka: Hrvatsko kemijsko društvo, 2025. str. 64-64
rijeka-pula-chem.hkd.hr
-
Duvnjak, Mirko; Vidović, Nikolina; Užarević, Krunoslav; Horvat, Gordan; Speranza, Giovanna; Tomišić, Vladislav; Cindro, Nikola | Mechanochemical chloride-templated synthesis of phenylalanine- and glycine-containing cyclopeptides // Book of Abstracts of 9th Faculty of Science PhD Student Symposium / Petrović, Petra (ur.). | Zagreb: Sveučilište u Zagrebu Prirodoslovno-matematički fakultet, 2025. str. 43-43
drive.google.com
-
Duvnjak, Mirko; Vidović, Nikolina; Užarević, Krunoslav; Horvat, Gordan; Speranza, Giovanna; Tomišić, Vladislav; Cindro, Nikola | Sustainable peptide cyclization: A solvent-free anion-templated mechanochemical approach // Abstract E-Book 20ACC. | Bangkok: The Chemical Society of Thailand, 2025. str. 394-394
acc2025thailand.com
Lisac, Katarina; Pongrac, Petra; Petrović Hađar, Emilija; Biliškov, Nikola; Avdoshenko, Stanislav; Popov, Alexey; Vujević, Lucija; Žilić, Dijana; Cvetnić, Marija; Bregović, Nikola et al. | Fullerene-templated discovery of novel zinc imidazolate (ZnIm₂) solid forms // Mech’cheM 2025: New forces in Mechanochemistry. | Montpellier: -, 2025. str. 66-66
Martinez, Valentina; Grgurić, Toni; Razum, Marta; Zgrablić, Goran; Senkić, Ana; Karadeniz, Bahar; Etter, Martin; Brekalo, Ivana; Arhangelskis, Mihails; Pavić, Luka et al. | Unlocking conductivity: mechanochemical insights into alkali metal coordination polymers // Mech’cheM 2025: New forces in Mechanochemistry. | Montpellier: -, 2025. str. 81-81
-
Užarević, Krunoslav | Thermal control of milling reactors for advancing preparative mechanochemistry // Solutions in Chemistry 2024: Book of Abstracts / Kassal, Petar; Meštrović, Ernest; Namjesnik, Danijel et al. (ur.). | Zagreb: Hrvatsko kemijsko društvo, 2024. str. 49-49
solutionsinchemistry.hkd.hr
-
Cindro, Nikola; Duvnjak, Mirko; Vidović, Nikolina; Užarević, Krunoslav; Horvat, Gordan; Speranza, Giovanna; Tomišić, Vladislav | Mechanochemical chloride-templated macrocyclization of oligopeptides // Solutions in Chemistry 2024: Book of Abstracts / Kassal, Petar; Meštrović, Ernest; Namjesnik, Danijel et al. (ur.). | Zagreb: Hrvatsko kemijsko društvo, 2024. str. 57-57
solutionsinchemistry.hkd.hr
-
Lisac, Katarina; Pantalon Junaj, Natalija; Mendaš, Dora; Martinez, Valentina; Karadeniz, Bahar; Ročnik Kozmelj, Tina; Đilović, Ivica; Grilc, Miha; Jasiukaitytė Grojzdek, Edita; Herak, Mirta et al. | Catalytic and magnetic study of monometallic and bimetallic MOF-74 materials with terephthalic and isophthalic linkers // Book of Abstracts Summer Conference of Croatian Chemical Society Rijeka - Pula 2024 / Rezić Meštrović, Iva; Vidović, Nikolina; Petković Didović, Mirna et al. (ur.). | Rijeka: Croatian Chemical Society, 2024. str. 10-10
rijeka-pula-chem.hkd.hr
Palčić, Ana ; Jurin, Mladenka ; Jakupec, Nikola ; Pantalon Juraj, Natalija ; Etter, Martin ; Užarević, Krunoslav | IN SITU INVESTIGATION OF ZEOLITE SYNTHESIS WHILE MILLING AT ELEVATED TEMPERATURES // Book of abstracts: 9th Conference of the Federation of European Zeolite Associations (FEZA 2023) / Zabukovec Logar, Nataša; Rakić, Vesna; Bronić, Josip (ur.). | Slovenia: Slovenian Zeolite Association, 2023. str. 41-41
-
Alić, Jasna; Etter, Martin; Rubčić, Mirta; Štefanić, Zoran; Šekutor, Marina; Užarević, Krunoslav; Stolar, Tomislav | Direct in situ measurement of polymorphic transition temperatures in thermo-mechanochemical reactions // Solid-State Science & Research 2023 : book of abstracts / Biliškov, Nikola; Karadeniz, Bahar; Juraj Pantalon, Natalija (ur.). | Zagreb: Institut Ruđer Bošković, 2023. str. 41-41
www.scires2023.irb.hr
-
Lisac, Katarina; Mendeš, Dora; Martinez, Valentina; Karadeniz, Bahar; Ročnik Kozmelj, Tina; Đilović, Ivica; Grilc, Miha, Jasiukaitytė-Grojzdek, Edita; Herak, Mirta; Likozar, Blaž; Žilić, Dijana et al. | Monometallic and bimetallic MOF-74 materials based on structural isomers as linkers // Solid-State Science & Research 2023 : book of abstracts / Biliškov, Nikola; Karadeniz, Bahar; Juraj Pantalon, Natalija (ur.). | Zagreb: Institut Ruđer Bošković, 2023. str. 44-44
www.scires2023.irb.hr
-
Grgurić, Toni; Martinez, Valentina; Etter, Martin; Užarević, Krunoslav | Green and scalable synthesis of alkali metal dhta polymers // Solid-State Science & Research 2023 : book of abstracts / Biliškov, Nikola; Karadeniz, Bahar; Juraj Pantalon, Natalija (ur.). | Zagreb: Institut Ruđer Bošković, 2023. str. 97-97
www.scires2023.irb.hr
-
Martinez, Valentina; Mendaš, Dora; Lisac, Katarina; Jurec, Jurica; Žilić, Dijana; Užarević, Krunoslav | Structural and magnetic study of MOF-74 isophthalic homologues // Solid-State Science & Research 2023 : book of abstracts / Biliškov, Nikola; Karadeniz, Bahar; Juraj Pantalon, Natalija (ur.). | Zagreb: Institut Ruđer Bošković, 2023. str. 93-93
www.scires2023.irb.hr
-
Pantalon Juraj, Natalija; Stolar, Tomislav; Cindro, Nikola; Hernández, José G.; Užarević, Krunoslav | Mineral and solid acid-catalyzed mechanochemical synthesis of esters // Solid-State Science & Research 2023 : book of abstracts / Biliškov, Nikola; Karadeniz, Bahar; Juraj Pantalon, Natalija (ur.). | Zagreb: Institut Ruđer Bošković, 2023. str. 80-80
fulir.irb.hr
Jakupec, Nikola; Uran, Erik; Etter, Martin; Užarević, Krunoslav; Palčić, Ana | Synthesis of CHA zeolite via thermally controlled mechanochemistry // Book of abstracts: 9th Conference of the Federation of European Zeolite Associations (FEZA 2023), 2nd-6th of July 2023, Portorož-Portorose, Slovenia / Zabukovec Logar, Nataša; Rakić, Vesna; Bronić, Josip (ur.). | Ljubljana: Slovenska akademija znanosti in umetnosti (SAZU), 2023. str. 207-207
-
Jakupec, Nikola; Uran, Erik; Etter, Martin; Užarević, Krunoslav; Palčić, Ana | Mechanochemical interzeolite conversion of FAU to CHA zeolite // 7th Faculty of Science PhD Student Symposium - Book of Abstracts / Pavlek, Katarina (ur.). | Zagreb: Prirodoslovno-matematički fakultet Sveučilišta u Zagrebu, 2023. str. 47-47
phdss.pmf.unizg.hr
-
Jurin, Mladenka ; Pantalon Juraj, Natalija ; Etter, Martin ; Užarević, Krunoslav ; Palčić, Ana | Monitoring thermo-milling of natural zeolite clinoptilolite // Book of abstracts: 9th Conference of the Federation of European Zeolite Associations (FEZA 2023) / Zabukovec Logar, Nataša; Rakić, Vesna; Bronić, Josip (ur.). | Slovenia: Slovenian Zeolite Association, 2023. str. 212-212
feza2023.org
-
Martinez, Valentina ; Muratović, Senada ; Karadeniz, Bahar ; Mali, Gregor ; Krupskaya, Yulia ; Kataev, Vladislav ; Žilić, Dijana ; Užarević, Krunoslav | Unraveling the structural changes of zinc-copper mofs by combining SS-NMR and EPR spectroscopy // 7th Adriatic NMR Conference: Book of Abstracts. | Zagreb: Hrvatsko kemijsko društvo, 2023. str. 51-51
adriatic-nmr-conference.hkd.hr
-
Vujević, Lucija ; Karadeniz, Bahar ; Žilić, Dijana ; Užarević, Krunoslav ; Kveder, Marina | Continuous-wave EPR study of MOF-525 and PCN-223 doped with various paramagnetic centers // Continuous-wave EPR study of mof-525 and PCN-223 doped with various paramagnetic centers. | Zagreb: Prirodoslovno-matematički fakultet Sveučilišta u Zagrebu, 2023. str. 129-129
phdss.pmf.unizg.hrdrive.google.com
-
Vujević, Lucija ; Karadeniz, Bahar ; Cindro Nikola ; Avdoshenko, Stanislav M. ; Popov, Alexey A. ; Žilić, Dijana ; Užarević, Krunoslav ; Kveder, Marina | Electron Spin Relaxation Times of Copper(II) Ions in MOF-525 and PCN-223 Measured by Pulsed EPR Spectroscopy // V. simpozij supramolekulske kemije : knjiga sažetaka = Supramolecular Chemistry 2022 : Book of Abstracts. | Zagreb: Institut Ruđer Bošković, 2022. str. 28-28
supramolchem.org
-
Martinez, Valentina ; Muratović, Senada ; Karadeniz, Bahar ; Pajić, Damir ; Krupskaya, Yulia ; Kataev, Vladislav ; Žilić, Dijana ; Užarević, Krunoslav | Mechanochemical route for the synthesis of magnetic copper-zinc MOF-74 materials // 7th Faculty of Science PhD Student Symposium : Book of Abstracts. | Zagreb: Prirodoslovno-matematički fakultet Sveučilišta u Zagrebu, 2023. str. 49-49
drive.google.comphdss.pmf.unizg.hr
-
Užarević, Krunoslav ; Martinez, Valentina ; Stolar, Tomislav ; Brekalo, Ivana ; Karadeniz, Bahar | Thermal control of milling reactors for new advances in preparative mechanochemistry // 10th International Conference on Mechanochemistry and Mechanical Alloying - Book of Abstracts / Delogu, Francesco ; Colacino, Evelina (ur.).. | 2022. str. 72-72
income2022.it
Talajić, Gregor ; Brekalo, Ivana ; Topić, Edi ; Cindro, Nikola ; Užarević, Krunoslav | Cyclobutanetetraamino acid derived homochiral metal- organic frameworks // 27th Croatian Meeting of Chemists and Chemical Engineers and 5th Symposium Vladimir Prelog : Book of Abstracts. | Zagreb, 2021. str. 247-247
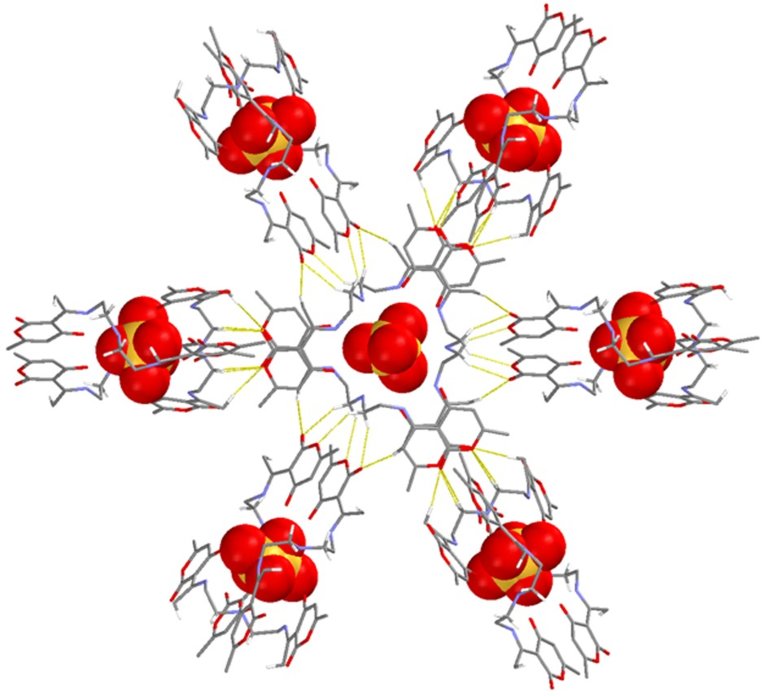
Karadeniz, Bahar ; Vujević, Lucija ; Žilić, Dijana ; Kveder, Marina ; Užarević, Krunoslav | Controllable synthesis and transformations of magnetic porphyrin-based zirconium MOFs and MOF composites by mechanochemistry // 10th International Conference on Mechanochemistry and Mechanical Alloying - Book of Abstracts. | Cagliari: Universita degli Studi di Cagliari, 2022. str. 56-56
-
Brekalo, Ivana ; Martinez, Valentina ; Karadeniz, Bahar ; Drapanauskaite, Donata ; Vriesema, Hein ; Stenekes, Robert ; Etter, Martin ; Dejanović, Igor ; Baltrusaitis, Jonas ; Užarević, Krunoslav | Scale-up of agrochemical urea-gypsum cocrystal synthesis using thermally-controlled mechanochemistry // The European Powder Diffraction Conference EPDIC 17: Book of Abstracts / Popović, Jasminka; Štefanić, Zoran (ur.). | Zagreb: Croatian Association of Crystallographers, 2022. str. 48-48
hukcac.wixsite.com
Ostojić, Tea ; Stolar, Tomislav ; Užarević, Krunoslav ; Meštrović, Ernest ; Grisanti, Luca | Hydrogen Bonding vs. π-stacking Interactions in Nucleobase Assemblies via Enhanced-Sampling MD // 12th European Symposium on Computing π-conjugated Compounds : Book of Abstracts. | 2022. str. 49-49
-
Ostojić, Tea ; Stolar, Tomislav ; Užarević, Krunoslav ; Meštrović, Ernest ; Grisanti, Luca | Nucleobase Assemblies via Enhanced- sampling MD: Understanding Biological Matter From Its Molecular Building Blocks / Samoudruživanje nukleinskih baza poboljšanim uzorkovanjem MD: razumijevanje biološke tvari iz perspektive molekulskih gradivnih blokova // Simpozij studenata doktorskih studija PMF-a : knjiga sažetaka = 6th Faculty of Science PhD student symposium. | Zagreb: Prirodoslovno-matematički fakultet Sveučilišta u Zagrebu, 2022. str. 278-279
www.pmf.unizg.hr
-
Martinez, Valentina ; Karadeniz, Bahar ; Biliškov, Nikola ; Lončarić, Ivor ; Popov, Alexey A. ; Užarević, Krunoslav | Mechanochemical strategy for controllable encapsulation of Buckminsterfullerene for preparation of fulleretic ZIF materials // The 28th Croatian-Slovenian Crystallographic Meeting : Book of Abstracts. | Zagreb: Hrvatska akademija znanosti i umjetnosti (HAZU) ; Hrvatska Kristalografska Zajednica, 2022. str. 18-18
cscm28.hazu.hr
-
Žilić, Dijana ; Muratović, Senada ; Martinez, Valentina ; Karadeniz, Bahar ; Pajić, Damir ; Brekalo, Ivana ; Arhangelskis, Mihails ; Mazaj, Matjaž ; Mali, Gregor ; Friščić, Tomislav et al. | Magnetism of copper(II)/zinc(II)-MOF-74 compounds obtained over three different synthetic pathways // 8th European Conference on Molecular Magnetism : Book of Abstracts. | Rennes, 2022. str. 113-113
filesender.renater.fr
Martinez, Valentina ; Brekalo, Ivana ; Karadeniz, Bahar ; Orešković, Patrik ; Drapanauskaite, Donata ; Dejanović, Igor ; Baltrusaitis, Jonas ; Užarević, Krunoslav | Scalable thermally-controlled mechanochemical synthesis of urea-gypsum agrochemical cocrystal // 10th International Conference on Mechanochemistry and Mechanical Alloying - Book of Abstracts. | Cagliari: Universita degli Studi di Cagliari, 2022. str. 111-111
Jakupec, Nikola ; J. Ardila-Fierro, Karen ; Martinez, Valentina ; Halasz, Ivan ; Etter, Martin ; Užarević, Krunoslav ; Palčić, Ana | Interzeolite conversion reactions of zeolite Y via thermally controlled mechanochemistry // 10th international conference on mechanochemistry and mechanical alloying : Book of Abstracts. | Cagliari: Universita Degli Studi di Cagliari, 2022. str. 99-99
-
Jakupec, Nikola ; J. Ardilla-Fierro, Karen ; Martinez, Valentina ; Halasz, Ivan ; Etter, Martin ; Užarević, Krunoslav ; Palčić, Ana | Termički kontrolirana mehanokemija za selektivne reakcije interzeolitne pretvorbe // 6. simpozij studenata doktorskih studija PMF-a : knjiga sažetaka = 6th Faculty of Science PhD student symposium : book of abstracts. | Zagreb: Prirodoslovno-matematički fakultet Sveučilišta u Zagrebu, 2022. str. 142-142
www.pmf.unizg.hr
-
Martinez, Valentina ; Stolar, Tomislav ; Prašnikar, Anže ; Karadeniz, Bahar ; Mali, Gregor ; Likozar, Blaž ; Užarević, Krunoslav | Selective conversion of CO2 to methanol over bimetallic MOF-74 catalyst // 6. simpozij studenata doktorskih studija PMF-a : knjiga sažetaka = 6th Faculty of Science PhD student symposium : book of abstracts. | Zagreb: Prirodoslovno-matematički fakultet Sveučilišta u Zagrebu, 2022. str. 70-71
www.pmf.unizg.hr
-
Muratović, Senada ; Martinez, Valentina ; Karadeniz, Bahar ; Pajić, Damir ; Krupskaya, Yulia ; Kataev, Vladislav ; Žilić, Dijana ; Užarević, Krunoslav | Magnetism of Cu(II)-MOF-74 and its three mixed analogous compounds with Zn(II) // 6. simpozij studenata doktorskih studija PMF-a : knjiga sažetaka = 6th Faculty of Science PhD student symposium : book of abstracts. | Zagreb: Prirodoslovno-matematički fakultet Sveučilišta u Zagrebu, 2022. str. 274-275
www.pmf.unizg.hr
-
Martinez, Valentina ; Muratović, Senada ; Karadeniz, Bahar ; Krupskaya, Yulia ; Kataev, Vladislav ; Žilić, Dijana ; Užarević, Krunoslav | EPR insight into magnetic properties of bimetallic Copper/Zinc MOF-74 materials / EPR uvid u magnetska svojstva dvometalnihbakar/cink MOF-74 materijala // XIV. Susret mladih kemijskih inženjera : knjiga sažetaka = XIV Meeting of Young Chemical Engineers : Book of Abstracts. | Zagreb: Hrvatsko društvo kemijskih inženjera i tehnologa (HDKI), 2022. str. 25-25
www.hdki.hr
Lucija Vujević ; Bahar Karadeniz ; Krunoslav Užarević ; Dijana Žilić ; Marina Kveder | Continuous-wave EPR study of MOF-525 and PCN-223 doped with copper(II) ion as the metal center // SCIRES2021 MEETING : Book of Abstracts. | Zagreb: Institut Ruđer Bošković, 2021, 62-111, 1
Žilić, Dijana ; Muratović, Senada ; Karadeniz, Bahar ; Martinez, Valentina ; Užarević, Krunoslav ; Pajić, Damir ; Krupskaya, Yilia ; Kataev, Vladislav | Magnetic properties of mixed copper/zinc MOF-74 compounds and their amorphous phases // Solid-State Science & Research 2021 : Book of Abstracts and Programme. | Zagreb: Institut Ruđer Bošković, 2021. str. 63-111
Užarević, Krunoslav ; Karadeniz, Bahar ; Martinez, Valentina ; Popov, Alexey ; Biliškov, Nikola | Controllable entrapment of fullerene into small- aperture MOF for preparation of tunable fulleretic materials // 27th Annual Meeting of the Slovenian Chemical Society : Book of Abstracts. | Ljubljana: Slovenian Chemical Society, 2021. str. 12-12
-
Vujević, Lucija ; Karadeniz, Bahar ; Užarević, Krunoslav ; Žilić, Dijana ; Kveder, Marina | Magnetic properties of MOF 525 and PCN 223 doped with copper (II) ions studied by EPR spectroscopy // 27th Croatian Meeting of Chemists and Chemical Engineers : Book of Abstracts. | Zagreb: Hrvatsko kemijsko društvo, 2021. str. 198-198
27hskiki.hkd.hr
-
Karadeniz, Bahar ; Martinez, Valentina ; Popov, Alexey ; Biliškov, Nikola ; Užarević, Krunoslav | Controllable entrapment of fullerene into small- aperture MOF for preparation of tunable fulleretic materials // 4th EuroMOF, European Conference on Metal Organic Frameworks and Porous Polymers : Programme & Book of abstracts. | Krakov: Deutsche Gesellschaft für chemisches Apparatewesen (DECHEMA), 2021, IS3, 1
app.pinetool.ai
Martinez, Valentina ; Stolar, Tomislav ; Prašnikar, Anže ; Karadeniz, Bahar ; Mali, Gregor ; Likozar, Blaž ; Užarević, Krunoslav | Mechanochemical amorphization of MOF-74 catalyst for selective conversion of CO2 to methanol // 27th Annual Meeting of the Slovenian Chemical Society : Book of Abstracts. | Ljubljana: Slovenian Chemical Society, 2021, 271, 1
-
Bregović, Nikola ; Barišić, Dajana ; Cindro, Nikola ; Vidović, Nikolina ; Užarević, Krunoslav ; Tireli, Martina ; Lešić, Filip ; Tomišić, Vladislav | Receptors based on sulfonylurea and amide functional groups – newcomers and veterans in anion-coordination chemistry // Adriatic NMR Conference 2021 : Book of abstracts. | Zagreb: Hrvatsko kemijsko društvo, 2021. str. 32-32
adriatic-nmr-conference.chem.pmf.hr
-
Martinez, Valentina ; Karadeniz, Bahar ; Užarević, Krunoslav | Mechanochemical strategy for controllable encapsulation of fullerene C60 within metal-organic framework ZIF-8 // 2nd International School on Porous Materials: MOFschool2021 : Book of Abstracts. | 2021. str. 56-56
iris.unito.it
Karadeniz, Bahar ; Martinez, Valentina ; Užarević, Krunoslav ; Popov, Alexey | Controllable Encapsulation of Fullerene-C60 into the small-aperture pore of ZIF-8 by Ball Milling // Solid State Science and Research 2021 : Book of Abstracts and Programme. | Zagreb: Institut Ruđer Bošković, 2021. str. 91-91
Martinez, Valentina ; Stolar, Tomislav ; Prašnikar, Anže ; Karadeniz, Bahar ; Likozar, Blaž ; Užarević, Krunoslav | Bimetallic MOF-74 Catalysts for Selective Hydrogenation of CO2 to Methanol // Solid State Science and Research : Book of Abstracts and Programme. | Zagreb: Institut Ruđer Bošković, 2021. str. 90-90
Biliškov, Nikola ; Milanović, Igor ; Užarević, Krunoslav | Preparation of materials for energy conversion and storage by controlled mechanochemistry // 25th Annual Green Chemistry & Engineering Conference. | Washington (MD): American Chemical Society (ACS), 2021, K5NY79HHBHS, 1
Milanović, Igor ; Biliškov, Nikola ; Užarević, Krunoslav ; Lukin, Stipe ; Halasz, Ivan | Mechanochemical Synthesis of Novel Mg- and Ca- Containing Bimetallic Amidoboranes // Solid State Science and Research 2021 : Book of Abstracts and Programme. | Zagreb: Institut Ruđer Bošković, 2021. str. 50-50
-
Martinez, Valentina ; Karadeniz, Bahar ; Užarević, Krunoslav | Efikasno i kontrolirano uklapanje buckminsterfullerena u ZIF-8 sodalitne topologije mehanokemijskim putem // 5.Simpozij studenata doktorskih studija PMF-a : Knjiga sažetaka = 5th PhD Student Symposium : Book of Abstracts. | Zagreb: Prirodoslovno-matematički fakultet Sveučilišta u Zagrebu, 2021. str. 156-157
www.pmf.unizg.hr
-
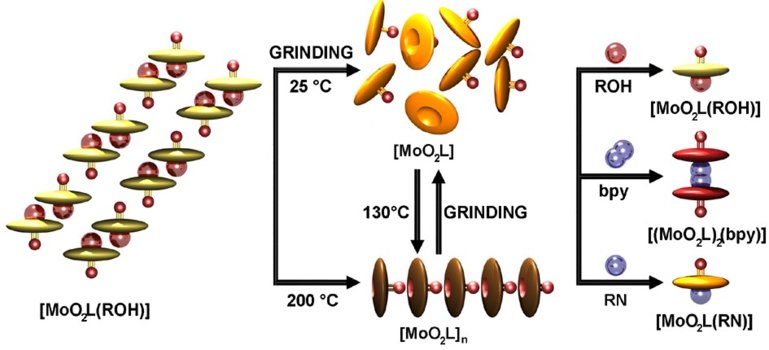
Užarević, Krunoslav ; Karadeniz, Bahar ; Stolar, Tomislav ; Martinez, Valentina | Rapid, clean and scalable synthesis of microporous functional MOFs and their non- conventional forms via mechanochemistry // CEMWOQ6p5 Program. | 2020. str. 33-33
friscic.research.mcgill.ca
-
Karadeniz, Bahar ; Užarević, Krunoslav ; Friščić, Tomislav ; Žilić, Dijana ; Muratović, Senada | Mechanochemical synthesis and topology transformation in polymorphic Zr-MOF qubit candidates // Crystal Engineering and Emerging Materials Workshop of Ontario and Quebec (CEMWOQ) : abstracts of posters. | 2020. str. 33-33
friscic.research.mcgill.ca
-
Muratović, Senada ; Karadeniz, Bahar ; Žilić, Dijana ; Užarević, Krunoslav | Istraživanje cirkonijevih MOF-ova s ugrađenim metalnim ionima EPR spektroskopijom // Knjiga sažetaka. | Zagreb, 2020. str. 117-117
www.pmf.unizg.hr
Karadeniz, Bahar ; Užarević, Krunoslav ; Žilić, Dijana ; Muratović, Senada | Mechanochemical synthesis of polymorphic metal-organic frameworks and their topology transformationION // XIII. susret mladih kemijskih inženjera : knjiga sažetaka. | Zagreb: Hrvatsko društvo kemijskih inženjera i tehnologa (HDKI), 2020. str. 45-45
-
Martinez, Valentina ; Karadeniz, Bahar ; Užarević, Krunoslav | Kontrolirano i učinkovito uklapanje C60- fulerena unutar sodalitne metal-organske mreže s malim aperturama // XIII. Susret mladih kemijskih inženjera : knjiga sažetaka. | Zagreb: Hrvatsko društvo kemijskih inženjera i tehnologa (HDKI), 2020. str. 173-173
www.hdki.hr
-
Bregović, Nikola ; Barišić, Dajana ; Cindro, Nikola ; Užarević, Krunoslav ; Tomišić, Vladislav | Complex equilibrium systems in supramolecular chemistry – „A blessing or a curse?” // III. Simpozij supramolekulske kemije : knjiga sažetaka. | Zagreb: Institut Ruđer Bošković, 2019. str. 3-3
supramolchem2019.irb.hr
-
Đilović, Ivica ; Užarević, Krunoslav | Selective binding of weakly coordinating anions: exploring the conformational space of flexible receptors // Abstracts of the 2017 American Crystallographic Association Meeting, Acta Crystallographica, A73. | 2017. str. a166-a166. doi: 10.1107/S010876731709835X
doijournals.iucr.orgdoi.org
-
Karadeniz, Bahar ; Stolar, Tomislav ; Užarević, Krunoslav | Mechanochemical Synthesis of Zirconium-based Metal Organic Frameworks // Solid-State Science & Research 2019, BOOK OF ABSTRACTS. | Zagreb: Institut Ruđer Bošković, 2019. str. 50-50
scires2019.irb.hrscires.irb.hr
-
Žilić, Dijana ; Muratović, Senada ; Karadeniz, Bahar ; Užarević, Krunoslav ; Herak, Mirta ; Krupskaya, Yulia ; Kataev, Vladislav | Ni-MOF-74 and its different phases ; structural, magnetization and ESR studies // Solid-State Science & Research 2019, Book of Abstracts, 27-29 June 2019, Zagreb, Croatia. | Zagreb: Institut Ruđer Bošković, 2019. str. 83-83
scires2019.irb.hr
-
Cindro, Nikola ; Barišić, Dajana ; Bregović, Nikola ; Užarević, Krunoslav ; Tomišić, Vladislav | Synthesis and photochemical properties of an anion receptor containing azocarboxamide groups // 26. Hrvatski skup kemičara i kemijskih inženjera s međunarodnim sudjelovanjem ; 4. Simpozij Vladimir Prelog : knjiga sažetaka. | Zagreb: Hrvatsko društvo kemijskih inženjera i tehnologa (HDKI), 2019. str. 136-136
www.hdki.hr
-
Žilić, Dijana ; Muratović, Senada ; Karadeniz, Bahar ; Užarević, Krunoslav ; Krupskaya, Yulia ; Kataev, Vladislav | Magneto-Structural Correlations in Ni(II)-MOF-74 and Intermediate Compounds Studied by HF-ESR Spectroscopy // ECMolS 2018 - 2nd European Conference on Molecular Spintronics. | Peniscola, 2018. str. 140-140
icmol.es
Bjelopetrović, Alen ; Lukin, Stipe ; Halasz, Ivan ; Užarević, Krunoslav ; Barišić, Dajana ; Juribašić Kulcsar, Marina, Ćurić, Manda | Mechanism of Solid-State C–H Bond Activation by Different Pd(II) Catalysts // XXVIII International Conference on Organometallic Chemistry ICOMC2018 : Book of abstracts. | Lahti, 2018. str. 181-181
Bjelopetrović, Alen ; Lukin, Stipe ; Halasz, Ivan ; Užarević, Krunoslav ; Đilović, Ivica ; Barišić, Dajana ; Budimir, Ana ; Juribašić- Kulcsar, Marina ; Ćurić, Manda | X-Ray Identification and Characterization of Azobenzene Palladacycles Obtained by Solid- State C–H Bond Activation // The Twenty-Sixth Croatian-Slovenian Crystallographic Meeting : Book of abstracts. | 2018. str. 46-46
Bjelopetrović, Alen ; Lukin, Stipe ; Halasz, Ivan ; Užarević, Krunoslav ; Đilović, Ivica ; Barišić, Dajana ; Budimir, Ana ; Juribašić- Kulcsar, Marina ; Ćurić, Manda | X-Ray Analysis of Intermediates and Products Involved in Solid-State C-H Bond Activation by Pd(II) Chloride Precoursors // The Twenty-Sixth Croatian-Slovenian Crystallographic Meeting : Book of abstracts. | 2018. str. 33-33
Bjelopetrović, Alen ; Lukin, Stipe ; Juribašić Kulcsar, Marina ; Halasz, Ivan ; Užarević, Krunoslav ; Ćurić, Manda | Aktivacija ugljik-vodik veze različitim paladijevim prekursorima u čvrstom stanju // XII Susret mladih kemijskih inženjera : knjiga sažetaka = XII Meeting of Young Chemical Engineers : Book of Abstracts. | Zagreb: Hrvatsko društvo kemijskih inženjera i tehnologa (HDKI), 2018. str. 46-46
Užarević, Krunoslav ; Fidelli, Athena ; Farha, Omar ; Friščić, TOmislav | Mechanochemical synthesis of highly-ordered microporous zirconium-based metal-organic frameworks // INCOME2017, 9th International Conference on Mechanochemistry and Mechanical Alloying: Program and Abstracts. | Košice, 2017. str. 5-5
Užarević, Krunoslav | Solvent-free methods for controllable synthesis of metastable pharmaceutical solids // 24th Congress and General Assembly of the International Union of Crystallography (IUCr 2017): E-Abstracts. | 2017. str. 898-898
-
Užarević, Krunoslav | Selektivno molekulsko prepoznavanje u čvrstom stanju // Minisimpozij Supramolekulska kemija u Hrvatskoj : dosezi i pogled u budućnost : Knjiga sažetaka = book of abstracts. | Zagreb: Institut Ruđer Bošković, 2017. str. 12-12
supramolchem.org
Užarević, Krunoslav ; Ferdelji, Nenad ; Halasz, Boris ; Halasz, Ivan | Monitoring the Heat Flow in Mechanochemical Reactions // Solid-State Science & Research : Book of Abstracts and Programme. | Zagreb: Institut Ruđer Bošković, 2017. str. 52-52
Lukin, Stipe ; Stolar, Tomislav ; Tireli, Martina ; Di Michiel, Marco ; Halasz, Ivan ; Užarević, Krunoslav | Solvent-free synthesis of pharmaceutically active compounds // The 1st European PhD & Postdoc Symposium : Book of Abstracts. | Barcelona, 2017. str. 129-129
Barišić, Dajana ; Cindro, Nikola ; Bregović, Nikola ; Frkanec, Leo ; Užarević, Krunoslav ; Tomišić, Vladislav | Sinteza i kompleksacijska svojstva aromatskih diurea // 2. Simpozij studenata kemičara : knjiga sažetaka. | Zagreb: Hrvatsko kemijsko društvo, 2015. str. 16-16
Lukin, Stipe ; Stolar, Tomislav ; Tireli, Martina ; V. Blanco, Maria ; Babić, Darko ; Friščić, Tomislav ; Užarević, Krunoslav ; Halasz, Ivan ; | Quantitative in situ monitoring of mechanochemical selectivity in pharmaceutical cocrystal polymorphs // NCOME2017, 9th International Conference on Mechanochemistry and Mechanical Alloying: Program and Abstracts. | Košice, 2017. str. 51-51
Halasz, Ivan ; Užarević, Krunoslav ; Lukin, Stipe ; Friščić, Tomislav | In situ monitoring as tools to study mechanochemical reactions // INCOME2017, 9th International Conference on Mechanochemistry and Mechanical Alloying: Program and Abstracts. | Košice, 2017. str. 48-48
Tireli, Martina ; Lukin, Stipe ; Stolar, Tomislav ; di Michiel, Marco ; Halasz, Ivan ; Užarević, Krunoslav | In situ monitoring and mechanism of the mechanochemical Knoevenagel reaction // 9th International Conference on Mechanochemistry and Mechanochemical Alloying (INCOME2017) : Program and Abstracts. | Košice: -, 2017. str. x-x
Biliškov, Nikola ; Halasz, Ivan ; Užarević, Krunoslav ; Grbović Novaković, Jasmina ; Milanović, Igor ; Lukin, Stipe ; Milošević, Sanja ; Borgschulte, Andreas ; Callini, Elsa | In-situ Raman Spectroscopic Monitoring of Ball Milling Preparations of Amidoboranes // INCOME2017, 9th International Conference on Mechanochemistry and Mechanical Alloying : Program and Abstracts. | Košice, 2017. str. 91-91
Stolar, Tomislav ; Lukin, Stipe ; Požar, Josip ; Užarević, Krunoslav ; Meštrović, Ernest ; Halasz Ivan | Solid-state chemistry and polymorphism of the nucleobase adenine // The Twenty-fourth Croatian-Slovenian Crystallographic : Meeting-Book of Abstracts. | Zagreb: Hrvatska akademija znanosti i umjetnosti (HAZU) ; Hrvatska kristalografska zajednica HAZU, 2016. str. 18-18
Lukin, Stipe ; Stolar, Tomislav ; Užarević, Krunoslav ; Halasz, Ivan | Mechanochemical cocrystal formation studied by in situ Raman spectroscopy // The Twenty-fourth Croatian-Slovenian Crystallographic Meeting : Book of Abstracts. | Zagreb: Hrvatska akademija znanosti i umjetnosti (HAZU) ; Hrvatska kristalografska zajednica HAZU, 2016. str. 14-14
Lukin, Stipe ; Stolar, Tomislav ; Tireli, Martina ; Blanco, Maria V. ; Babić, Darko ; Friščić, Tomislav ; Halasz, Ivan ; Užarević, Krunoslav | Quantitative in situ monitoring of mechanochemical polymorph selectivity // Solid-State Science and Research 2017 : Book of Abstracts. | Zagreb: Institut Ruđer Bošković, 2017. str. 54-54
Bjelopetrović, Alen ; Barišić, Dajana ; Lukin, Stipe ; Halasz, Ivan ; Užarević, Krunoslav ; Juribašić Kulcsar, Marina ; Ćurić, Manda | Mechanosynthesis of 4, 4'-bis(N, N-dimethylamino)-azobenzene and its dicyclopalladated complex // Solid-State Science & Research : Book of Abstracts and Programme. | Zagreb: Institut Ruđer Bošković, 2017. str. 62-62
Juribašić Kulcsar, Marina ; Halasz, Ivan ; Budimir, Ana ; Užarević, Krunoslav ; Lukin, Stipe ; Ćurić, Manda | Reversible gas-solid ammonia N–H bond activation mediated by an organopalladium complex // Solid-State Science & Research : Book of Abstracts and Programme. | Zagreb: Institut Ruđer Bošković, 2017. str. 57-57
Žilić, Dijana ; Užarević, Krunoslav ; Friščić, Tomislav ; Dragović, Jure ; Pajić, Damir ; Krupskaya, Yulia ; Kataev, Vladislav | Magnetic properties of monometallic and bimetallic MOF-74 compounds studied by high-field ESR spectroscopy // Solid-State Science & Research : Book of Abstracts and Programme. | Zagreb: Institut Ruđer Bošković, 2017. str. 39-39
-
Tireli, Martina ; Lešić, Filip ; Bregović, Nikola ; Užarević, Krunoslav | Mechanochemical synthesis of amide-based supramolecular anion receptors // Adriatic NMR 2017 : Book of Abstracts. | Zagreb: Kemijski odsjek Prirodoslovno-matematičkog fakulteta Sveučilišta u Zagrebu ; Geološki odsjek Prirodoslovno-matematičkog fakulteta Sveučilišta u Zagrebu, 2017. str. 40-40
adriatic-nmr-conference.chem.pmf.hr
Tireli, Martina ; Lukin, Stipe ; di Michael, Marco ; Halasz, Ivan ; Užarević, Krunoslav | Mechanhanistic study of mechanochemical Knoevenagel reaction // Solid-State Science & Research : book of abstracts and programme. | Zagreb: Institut Ruđer Bošković, 2017. str. 83-83
-
Bregović, Nikola ; Tireli, Martina ; Lešić, Filip ; Užarević, Krunoslav ; Tomišić, Vladislav | Aromatic mono- and bis-amide derivatives as anion receptors in solution // 25. Hrvatski skup kemičara i kemijskih inženjera s međunarodnim sudjelovanjem ; 3. simpozij Vladimir Prelog : knjiga sažetaka = 25th Croatian Meeting of Chemist and Chemical Engineers with international participation. 3rd symposium “Vladimir Prelog” : Book of Abstracts. | Zagreb: Hrvatsko kemijsko Društvo, 2017. str. 71-71
drive.google.comwww.hdki.hr
Užarević, Krunoslav ; Ayoub, Ghada ; Halasz, Ivan ; Friščić, Tomislav | Clean and rapid synthesis of microporous mixed- metal MOF-74 materials via mechanochemistry // 24th Croatian-Slovenian Crystallographic Meeting, Book of Abstracts. | 2016. str. 52-52
Juribašić Kulcsar, Marina ; Užarević, Krunoslav ; Halasz, Ivan ; Ćurić, Manda | A tale of cyclopalladated azobenzene acetates // The Twenty-fourth Croatian-Slovenian Crystallographic Meeting Book of Abstracts / Bijelić, Mirjana ; Cetina, Mario ; Čobić, Andrea et al. (ur.). | Zagreb: Hrvatska udruga kristalografa, 2016. str. 39-39
Bregović, Nikola ; Cindro, Nikola ; Frkanec, Leo ; Užarević, Krunoslav ; Tomišić, Vladislav | Thermodynamic study of anion complexation by urea-, thiourea- and amide- based receptors in solution. | 2015. str. ---
Bregović, Nikola ; Cindro, Nikola ; Frkanec, Leo ; Užarević, Krunoslav ; Tomišić, Vladislav | Anion binding study of urea-, thiourea- and amide- based receptors by NMR spectroscopy // Pharma NMR Conference: Application of NMR Spectroscopy in Pharmaceutical Industry : Programme & Book of Abstracts. | Zagreb: International Association of Physical Chemists (IAPC), 2015. str. 33-33
Barišić, Dajana ; Cindro, Nikola ; Bregović Nikola ; Frkanec, Leo ; Užarević, Krunoslav ; Tomišić, Vladislav | Complexation properties of aromatic diureas // Pharma NMR Conference : Application of NMR Spectroscopy in Pharmaceutical Industry : Programme & Book of Abstract. | Zagreb: International Association of Physical Chemists (IAPC), 2015. str. 17-17
Jednačak, Tomislav ; Novak, Predrag ; Užarević, Krunoslav ; Bratoš, Igor ; Marković, Jelena ; Cindrić, Marina | Phenylenediamine derivatives of dehydroacetic acid: synthesis, characterisation and deuterium isotope effects // Pharma NMR Conference: Application of NMR Spectroscopy in Pharmaceutical Industry : Programme & Book of Abstracts. | Zagreb: International Association of Physical Chemists (IAPC), 2015. str. 27-27
-
Barišić, Dajana ; Cindro, Nikola ; Bregović, Nikola ; Frkanec, Leo ; Užarević, Krunoslav ; Tomišić, Vladislav | Synthesis and complexation properties of aromatic diureas and phenantroline ditopic receptor // 24. Hrvatski skup kemičara i kemijskih inženjera : knjiga sažetaka. | Zagreb: Hrvatsko društvo kemijskih inženjera i tehnologa (HDKI), 2015. str. 170-170
www.hdki.hr
-
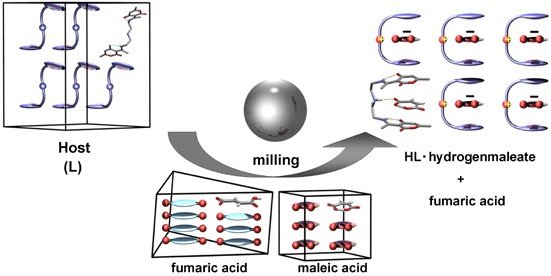
Užarević, Krunoslav ; Halasz, Ivan ; Đilović, Ivica ; Rubčić, Mirta ; Bregović, Nikola | Selective Mechanochemistry: Flexible Molecular Receptors for Recognition of Organic Isomers in Milling Processes // Past, Present and Future of Crystallography@Politecnico di Milano: From Small Molecules to Macromolecules and Supramolecular Structures. | Milano: Politechnico di Milano, 2013. str. 68-68
www.iycr2014.org
-
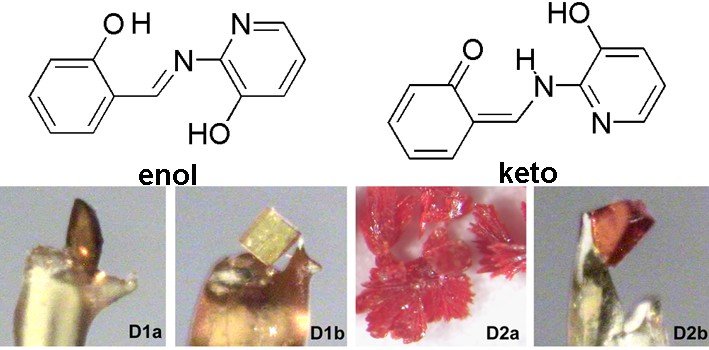
Đilović, Ivica ; Rubčić, Mirta ; Užarević, Krunoslav ; Halasz, Ivan ; Bregović, Nikola ; Mališ, Momir ; Kokan, Zoran ; Stein, Robin S. ; Dinnebier, Robert E. ; Tomišić, Vladislav. | Desmotropy, polymorphism and solid-state proton transfer: four solid forms of an aromatic o-hydroxy Schiff base // Past, Present and Future of Crystallography@ Politecnico di Milano: From Small Molecules to Macromolecules and Supramolecular Structures. | Milano: Politecnico di Milano, 2013. str. 51-51
www.iycr2014.org
Bregović, Nikola ; Užarević, Krunoslav ; Nemec, Vinko ; Tomišić, Vladislav | An Enaminone Derivative of Dehydroacetic Acid as an Effective Anion Receptor in Methanol and Acetonitrile Solutions // E-WISPOC : Book of Abstracts. | Padova: Cooperativa Libraria Editrice Università di Padova (CLEUP), 2013. str. 551-551
Bregović, Nikola ; Užarević, Krunoslav ; Cindrić, Marina ; Tomišić, Vladislav | A Cis-Trans Selective Anion Receptor - Solving The Solution // YoungChem - 9. International Congress of Young Chemists : Book of Abstracts. | Varšava: Chemical Scientific Society, 2011. str. 92-92
-
Bregović, Nikola ; Užarević, Krunoslav ; Cindrić Marina ; Tomišić Vladislav | Enaminonski derivat dehidracetne kiseline kao receptor aniona u otopini // XXII. Hrvatski skup kemičara i kemijskih inženjera : knjiga sažetaka. | Zagreb: Hrvatsko društvo kemijskih inženjera i tehnologa (HDKI), 2011. str. 114-114
www.hdki.hr
Jednačak, Tomislav ; Novak, Predrag ; Užarević, Krunoslav ; Bratoš, Igor ; Marković, Jelena ; Cindrić, Marina | Synthesis and characterization of deuterated isotopomers of bioactive phenylenediamine derivatives of 3-acetyl-4-hydroxy-6-methyl-2H- pyran-2-dione // XXII. Hrvatski skup kemičara i kemijskih inženjera - Knjiga Sažetaka. | Zagreb: Hrvatsko društvo kemijskih inženjera i tehnologa (HDKI), 2011. str. 145-145
Užarević, Krunoslav ; Đilović, Ivica ; Cindrić, Marina ; Matković-Čalogović, Dubravka | Conformational adaptations of podands as a base for selective binding of stereoisomers // 25th European Crystallographic Meeting Abstracts. | Istanbul, 2009. str. 257-257
UŽAREVIĆ, Krunoslav | FLEXIBLE PODANDS IN ANION RECEPTOR CHEMISTRY // Knjiga sažetaka, XXI. HRVATSKI SKUP KEMICARA I KEMIJSKIH INŽENJERA. | Zagreb: HKDI, 2009. str. 28-28
Užarević Krunoslav ; Rubčić Mirta ; Đilović Ivica ; Cindrić Marina. | Solid state study of three pseudopolymorphic forms of flexible tripodal host for complex oxoanions // 6th International Congress of Young Chemists "Young Chem 2008", Book of Abstracts. | Varšava, 2008. str. 162-162
Pičuljan, Katarina ; Novak, Predrag ; Užarević, Krunoslav ; Cindrić, Marina ; Hrenar, Tomica ; Jednačak, Tomislav ; Smrečki, Vilko | Hydrogen Bonding and Deuterium Isotope Effects in 13C NMR Spectra of Phenylene Enaminones Derived from Dehydroacetic Acid // EUCMOS 2008, XXIX European Congress on Molecular Spectroscopy, Book of Abstracts. | Opatija, 2008. str. 83-83
Užarević, Krunoslav ; Đilović, Ivica ; Kokan, Zoran ; Cindrić, Marina ; Matković-Čalogović, Dubravka. | Concomitant trimorph of cis-dioxo[(N-3-oxypirid-2-yl)-salicydeneiminato- O, N, O'-methanol] molybdenum(VI) // Seventeenth slovenian-croatian crystallographic meeting : Book of Abstracts and progamme = sedemnajsto slovensko-hrvatsko srečanje kristlografov : povzetki in program. | Ljubljana, 2008. str. 35-35
Matković-Čalogović, Dubravka; Užarević, Krunoslav; Đilović, Ivica; Cindrić, Marina | Flexible supramolecular ring-like host for various tetrahedral anion guests // Book of Abstracts, 24th European Crystallographic Meeting, ECM24. | Marakeš, 2007. str. 173-173
Matković-Čalogović, Dubravka; Užarević, Krunoslav; Cindrić, Marina; Đilović, Ivica | Flexible supramolecular host for trigonal and pyramidal anion guests // 16th Croatian-Slovenian Crystallographic Meeting, Book of abstracts. | Zagreb: Hrvatska akademija znanosti i umjetnosti (HAZU), 2007. str. 60-60
Užarević, Krunoslav ; Cindrić, Marina ; Đilović, Ivica ; Matković-Čalogović, Dubravka ; Šišak, Dubravka | Sinteza novih kompleksnih spojeva Mo(VI), Mo(V) i V(IV) s derivatima 3-acetil-4-hidroksi-6-metil-2-pirona // XX. jubilarni skup kemičara i kemijskih inženjera : Knjiga sažetaka. | Zagreb: Hrvatsko društvo kemijskih inženjera i tehnologa (HDKI), 2007. str. 84-84
Pičuljan, Katarina ; Marković, Jelena ; Užarević, Krunoslav ; Hrenar, Tomica ; Cindrić, Marina ; Meić, Zlatko ; Novak, Predrag | Vodikove veze i deuterijski izotopni efekti u 13C NMR spektrima N, N'-fenilen-diilbis[3-(1-aminoetil)-6-metil-2H-piran-2, 4(3H)-diona] // XX. jubilarni hrvatski skup kemičara i kemijskih inženjera : Knjiga sažetaka. | Zagreb: Hrvatsko društvo kemijskih inženjera i tehnologa / Kemija u industriji, 2007. str. 105-105
Užarević, Krunoslav ; Cindrić, Marina ; Kajfež Novak, Tanja | Synthesis of novel pyridopyrimidine derivatives and their complexes with dioxomolybdenum(VI) and oxovanadium(IV) // Book of Abstracts, CHTB 2006. | Krakov: Drukarnia, Uniwersytetu Jagiellonskiego, 2006. str. P-42-x
Cindrić, Marina ; Užarević, Krunoslav ; Kajfež Novak, Tanja ; Novak, Predrag | Kompleksni spojevi molibdena(VI) s makrocikličkim imino-ligandima // Knjiga sažetaka : 19. hrvatski skup kemičara i kemijskih inženjera. | Zagreb: Sveučilišni vjesnik, 2005. str. 51-51-x
Kajfež, Tanja ; Cindrić, Marina ; Užarević, Krunoslav ; Kamenar, Boris | Crystal and molecular structures of two new macrocyclic imine ligands derived from dehydroacetic acid // Twelfth Croatian-Slovenian crystallographic meeting : Book of abstracts. | Zagreb: Hrvatska akademija znanosti i umjetnosti (HAZU), 2003. str. 45-45
Popović, Zora ; Roje, Vibor ; Pavlović, Gordana ; Matković-Čalogović, Dubravka ; Užarević, Krunoslav ; Milić, Dalibor ; Đilović, Ivica | N-hydroxyphenyl structural isomers of 5-methoxysalicylaldimines and their zinc(II) and mercury(II) compounds // Sažetci. | Koprivnica: Hrvatsko društvo kemijskih inženjera i tehnologa (HDKI) ; Hrvatsko kemijsko drustvo, 2001. str. A16-x