Understanding cell entry pathway of Adenovirus type 26: way of improving vaccine vectors
Principal investigator
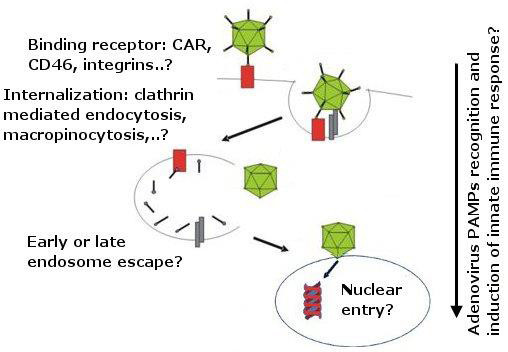
Adenovirus (AdV) based vectors have potential translational and commercial values and are currently studied as vectors for DNA transfer and vaccination. Adenovirus type 26 (AdV26) has fundamental differences in immunogenicity from other AdV vectors, which might give it advantage over other AdV serotypes in vaccine vector development. To the best of our knowledge there are no reports linking AdV26 entry pathway with induction of innate immune response, underlining the need for studying infection pathway used by AdV26. Accordingly, the main objectives of this study are investigating AdV26 receptor usage, binding, internalization, intracellular trafficking of AdV26 and AdV26 mediated induction of innate immune response inin vitrosettings.

