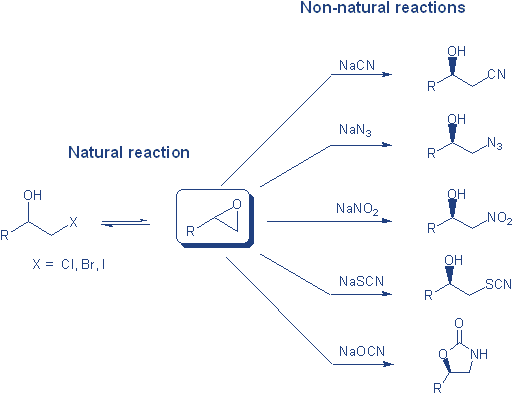
In recent years biocatalytic processes have found increasingly widespread application, particularly in the pharmaceutical and agrochemical industries where the need for optically pure molecules is high. Until recently biocatalysis was applied primarily to hydrolytic and esterification reaction. Several new classes of reaction are now also being exploited. Halohydrin dehalogenases recently emerged as particularly interesting enzymes as they catalyze several non-natural reactions. 1 They are bacterial enzymes and their natural role is the metabolism of vicinal haloacohols to form epoxides. It has been found that in the reverse reaction, epoxide ring opening, several non-natural anionic nucleophiles can be accepted, including azide, cyanide, nitrite and cyanate. 2
From the standpoint of an organic chemist, the ring opening reaction is much more intriguing and represent an excellent method to furnish enantiopure 1,2-azidoalcohols,3 1,2-cyanoalcohols,4,5 1,2-nitroalcohols6 and 2-oxazolidinones 7 respectively, from racemic epoxides through a kinetic resolution process.
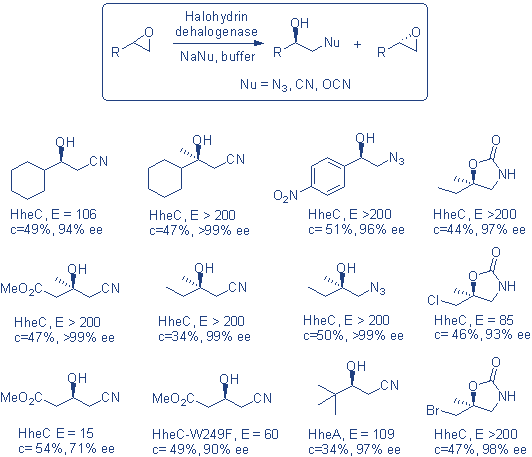
To access the b-substituted alcohols and 2-oxazolidinones, the procedure simply involves mixing racemic epoxide with 0.5-1 equivalent of sodium salt of nucleophile in buffer (pH 7.5) and typically 1-3 w% of the purified enzyme, with no other solvent required. The epoxide concentrations can go up to 300 mM. A broad range of sterically and electronically different epoxides can be resolved in >99% ee and yields of typically 40-45%.
Our ongoing investigations on halohydrin dehalogenases focus on:
- exploring the biocatalytic properties of wild-type and mutant enzymes
- organic solvents and gels as reaction media for enzyme catalysed reactions
- synthesis of 2-substituted oxazolidinones by kinetic resolution of epoxides
- dynamic kinetic resolution of epoxides
Selected publications:
- D. B. Janssen, M. Majerić Elenkov, G. Hasnaoui, B. Hauer, and J. H. Lutje Spelberg, Biochem. Soc. Trans. 34 (2006) 291-295.
- (a) J. H. Lutje Spelberg, L. Tang, M. van Gelder, R. M. Kellogg, and D. B. Janssen, Tetrahedron: Asymmetry 13 (2002) 1083-1089. (b) G. Hasnaoui, M. Majerić Elenkov, J. H. Lutje Spelberg, B. Hauer, and D. B. Janssen, ChemBioChem. 9 (2008) 1048-1051.(c) J. H. Lutje Spelberg, and D. B. Janssen, "Enzymatic conversion of epoxides" EP1287155B1.
- J. H. Lutje Spelberg, J. E. T. van Hylckama Vlieg, L. Tang, D. B. Janssen, and R. M. Kellogg, Org. Lett. 3 (2000) 41-43.
- M. Majerić Elenkov, B. Hauer, and D. B. Janssen, Adv. Synth. Catal. 348 (2006) 579-585.
- (a) M. Majerić Elenkov, L. Tang, B. Hauer, and D. B. Janssen, Adv. Synth. Catal. 349 (2007) 2279-2285.(b) B. Hauer, M. Majerić Elenkov, and D. B. Janssen, "A process for the production of an optically enriched tertiary alcohol", PCT/EP2006/069626.
- G. Hasnaoui, J. H. Lutje Spelberg, E. de Vries, and D. B. Janssen, Tetrahedron: Asymmetry 16 (2005) 1685-1692.
- (a) M. Majerić Elenkov, L. Tang, B. Hauer, and D. B. Janssen, Org. Lett. 10 (2008) 2417-2420. (b) B. Hauer, M. Majerić Elenkov, and D. B. Janssen, "Process for the preparation of optically active 5-substituted 2-oxazolidinones from racemic epoxides and cyanate employing a halohydrin dehalogenase", PCT/EP2007/051861.
- G. Hasnaoui-Dijoux, M. Majerić Elenkov, J.H. Lutje Spelberg, B. Hauer, and D.B. Janssen, "Catalytic Promiscuity of Halohydrin Dehalogenase and its Application in Enantioselective Epoxide Ring Opening", ChemBioChem 9 (2008) 1048–1051.
- M. M. Elenkov, I. Primožič, T. Hrenar, A. Smolko, I. Dokli, B. Salopek-Sondi, and L. Tang, "Catalytic activity of halohydrin dehalogenases towards spiroepoxides", Org. Biomol. Chem. 10 (2012) 5063–5072.
- L. Tang, X. Zhu, H. Zheng, R. Jiang, and M. M. Elenkov, "Key Residues for Controlling Enantioselectivity of Halohydrin Dehalogenase from Arthrobacter sp. Strain AD2, Revealed by Structure-Guided Directed Evolution", Appl. Environ. Microbiol. 78 (2012) 2631–2637.