The main focus of research in the Group for Polycyclic Chemistry is exploration of diamondoids, naturally occurring cage hydrocarbons that have unique properties and are potential building blocks for advanced materials and in supramolecular chemistry. These sp3-hybridized cyclic alkanes have the spatial arrangement of their carbon atoms comparable to a diamond crystal lattice and the smallest representative of diamondoid compounds is adamantane. Unlike bulk diamond, diamondoids can be selectively functionalized using various tools of synthetic organic chemistry. Consequently, a bottom-up design principle can be applied when developing new nanomaterials based on diamondoid cages.
In our group we combine experimental synthetic methods with computational tools in the design of new functionalized diamondoid derivatives, with a goal of applying the obtained scaffolds as novel advanced materials and as supramolecular systems. Specifically, our research is focused on cage functionalization, preparation of bigger diamondoid systems linked with various spacers and introduction of function-oriented subunits on the cage framework. We consider the synergy of computational and preparative approaches to be crucial for understanding the processes underlying the behavior and supramolecular properties of nanomaterials and therefore combine them when constructing new cage scaffolds.
Nataša Burić, dipl. ing.
Dr. Jasna Alić Stolar
|
A. A. Fokin, M. Šekutor, P. R. Schreiner, The Chemistry of Diamondoids. Building Blocks for Ligands, Catalysts, Materials, and Pharmaceuticals, 1st Edition, 2024, Wiley-VCH, Weinheim, ISBN: 978-3-527-34391-1. |
|
|
|
|
|
|
|
24. M. Alešković, J. Alić, W. E. Ernst, M. Šekutor, Self-Assembly of Diamondoid Clusters in Helium Nanodroplets Driven by Non-Covalent Interactions, J. Org. Chem. 2025, 90, 8825–8834. |
|
|
|
|
|
|
|
23. N. Burić, D. Loru, J. Alić, M. Šekutor, M. Schnell, P. Pinacho, Elucidating the structures of substituted adamantyl esters and ethers using rotational spectroscopy and computations, ChemPhysChem 2025, 26, e202500035. |
|
|
|
|
|
|
|
22. T. Šumanovac, M. Jakić, K. Mlinarić-Majerski, M. Šekutor, Synthesis, Alkali Metal Cation Binding and Computational Analysis of Adamantane-substituted Hexaaza Crown Ethers, Croat. Chem. Acta 2025, 98, DOI:10.5562/cca4146 |
|
|
|
|
|
|
|
21. J. Alić, I. Lončarić, M. Etter, M. Rubčić, Z. Štefanić, M. Šekutor, K. Užarević, T. Stolar, Direct in situ measurement of polymorphic transition temperatures under thermo-mechanochemical conditions, Phys. Chem. Chem. Phys. 2024, 26, 4840–4844. |
|
|
|
|
|
|
|
20. M. Alešković, M. Šekutor, Overcoming barriers with non-covalent interactions: Supramolecular recognition of adamantyl cucurbit[n]uril assemblies for medical applications, RSC Med. Chem. 2024, 15, 433–471. |
|
|
|
|
|
|
|
19. A. Usenik, M. Alešković, S. Roca, I. Markuš, M. Šekutor, J. Požar, Hosting of diamantane alcohols in water and hydrogen-bonded organic solvents: the (non-)classical hydrophobic effect, New. J. Chem. 2023, 47, 18745–18755. |
|
|
|
|
|
|
|
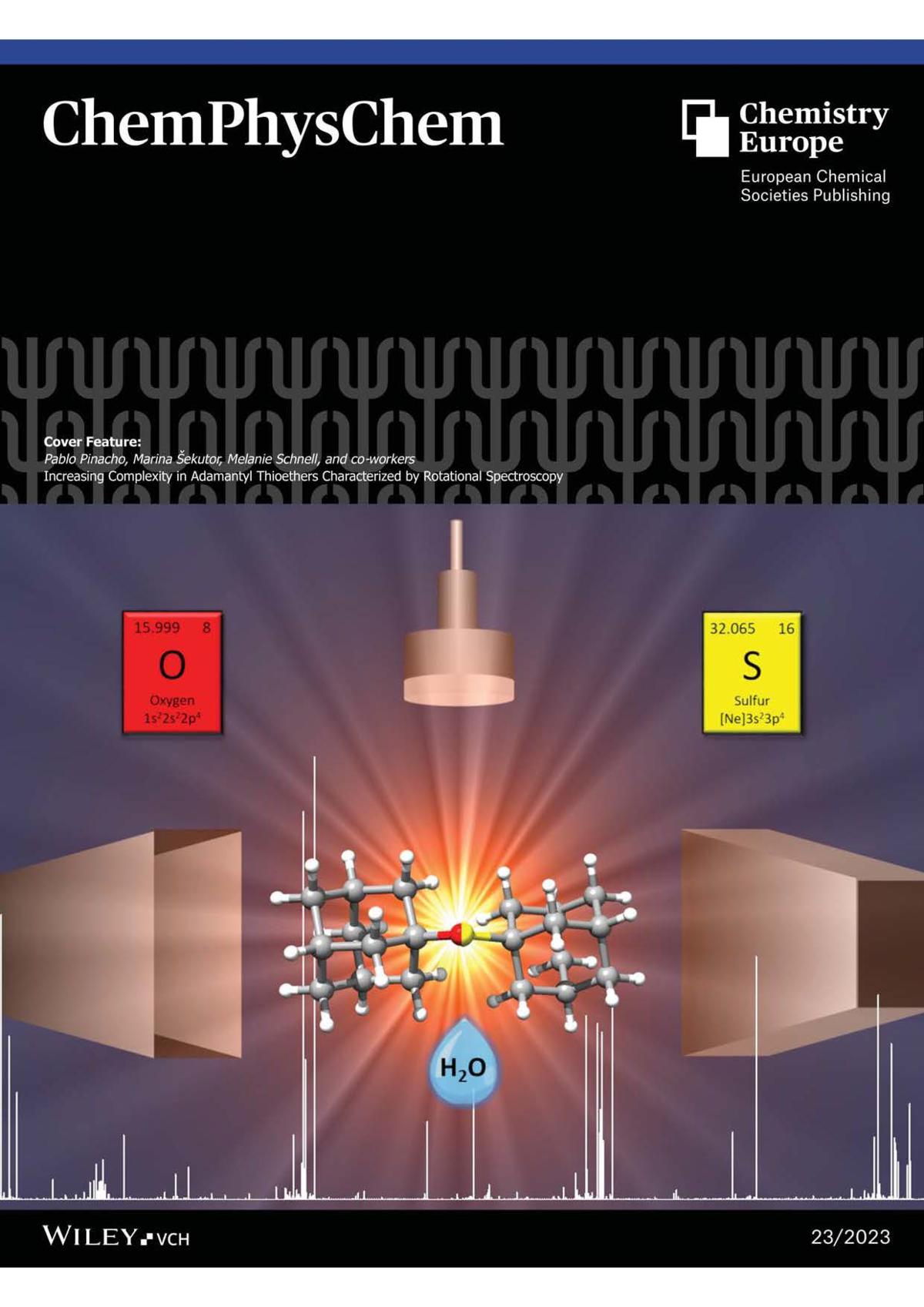
18. P. Pinacho, D. Loru, T. Šumanovac, M. Šekutor, M. Schnell, Increasing complexity in adamantyl thioethers characterized by rotational spectroscopy, ChemPhysChem 2023, 24, e2023005. |
|
|
|
|
|
|
|
17. M. Alešković, F. Küstner, R. Messner, F. Lackner, W. E. Ernst, M. Šekutor, Nanostructured supramolecular networks from self-assembled diamondoid molecules at ultracold conditions, Phys. Chem. Chem. Phys. 2023, 25, 17869–17876. |
|
|
|
|
|
|
|
16. J. Alić, R. Messner, M. Alešković, F. Küstner, M. Rubčić, F. Lackner, W. E. Ernst, M. Šekutor, Diamondoid ether clusters in helium nanodroplets, Phys. Chem. Chem. Phys. 2023, 25, 11951–11958. |
|
|
|
|
|
|
|
15. D. King, T. Šumanovac, S. Murkli, P. R. Schreiner, M. Šekutor, L. Isaacs, Cucurbit[8]uril Forms Tight Inclusion Complexes with Cationic Triamantanes, New J. Chem. 2023, 47, 5338–5346. |
|
|
|
|
|
|
|
14. J. Alić, T. Stolar, Z. Štefanić, K. Užarević, M. Šekutor, Sustainable Synthesis of Diamondoid Ethers by High-Temperature Ball Milling, ACS Sustainable Chem. Eng. 2023, 11, 617–624. |
|
|
|
|
|
|
|
13. M. Alešković, S. Roca, R. Jozepović, N. Bregović, M. Šekutor, Unravelling Binding Effects in Cyclodextrin Inclusion Complexes with Diamondoid Ammonium Salt Guests, New. J. Chem. 2022, 46, 13406–13414. |
|
|
|
|
|
|
|
12. J. Alić, I. Biljan, Z. Štefanić, M. Šekutor, Preparation and characterization of non-aromatic ether self-assemblies on a HOPG surface, Nanotechnology 2022, 33, 355603. |
|
|
|
|
|
|
|
11. J. Alić, R. Messner, F. Lackner, W. E. Ernst, M. Šekutor, London dispersion dominating diamantane packing in helium nanodroplets, Phys. Chem. Chem. Phys. 2021, 23, 21833–21839. |
|
|
|
|
|
|
|
10. M. M. Quesada Moreno, P. Pinacho, C. Pérez, M. Šekutor, P. R. Schreiner, M. Schnell, Do Docking Sites Persist Upon Fluorination? The Diadamantyl Ether-Aromatics Challenge for Rotational Spectroscopy and Theory, Chem. Eur. J. 2021, 27, 6198–6203. |
|
|
|
|
|
|
|
9. M. M. Quesada Moreno, P. Pinacho, C. Pérez, M. Šekutor, P. R. Schreiner, M. Schnell, London Dispersion and Hydrogen-Bonding Interactions in Bulky Molecules: The Case of Diadamantyl Ether Complexes, Chem. Eur. J. 2020, 26, 10817–10825. |
|
|
|
|
|
|
|
8. H.-Y. Gao, M. Šekutor, L. Liu, A. Timmer, H. Schreyer, H. Mönig, S. Amirjalayer, N. A. Fokina, A. Studer, P. R. Schreiner, H. Fuchs, Diamantane Suspended Single Copper Atoms, J. Am. Chem. Soc. 2019, 141, 315–322. |
|
|
|
|
|
|
|
7. D. Ebeling,1 M. Šekutor,1 M. Stiefermann, J. Tschakert, J. E. P. Dahl, R. M. K. Carlson, A. Schirmeisen, P. R. Schreiner, Assigning the absolute configuration of single aliphatic molecules by visual inspection, Nat. Commun. 2018, 9, 2420. |
|
|
|
|
|
|
|
6. D. Ebeling,1 M. Šekutor,1 M. Stiefermann, J. Tschakert, J. E. P. Dahl, R. M. K. Carlson, A. Schirmeisen, P. R. Schreiner, London Dispersion Directs On-Surface Self-Assembly of [121]Tetramantane Molecules, ACS Nano 2017, 11, 9459–9466. |
|
|
|
|
|
|
|
5. D. Sigwalt, M. Šekutor, L. Cao, P. Y. Zavalij, J. Hostaš, H. Ajani, P. Hobza, K. Mlinarić-Majerski, R. Glaser, L. Isaacs, Unraveling the Structure–Affinity Relationship between Cucurbit[n]urils (n = 7, 8) and Cationic Diamondoids, J. Am. Chem. Soc. 2017, 139, 3249–3258. |
|
|
|
|
|
|
|
4. J. Hostaš, D. Sigwalt, M. Šekutor, H. Ajani, M. Dubecký, J. Řezáč, P. Y. Zavalij, L. Cao, C. Wohlschlager, K. Mlinarić-Majerski, L. Isaacs, R. Glaser, P. Hobza, A Nexus between Theory and Experiment: Non-empirical Quantum Mechanical Computational Methodology Applied to Cucurbit[n]uril•Guest Binding Interactions, Chem. Eur. J. 2016, 22, 17226‒17238. |
|
|
|
|
|
|
|
3. O. Moncea, M. A. Gunawan, D. Poinsot, H. Cattey, J. Becker, R. I. Yurchenko, E. D. Butova, H. Hausmann, M. Šekutor, A. A. Fokin, J.-C. Hierso, P. R. Schreiner, Defying Stereotypes with Nanodiamonds: Stable Primary Diamondoid Phosphines, J. Org. Chem. 2016, 81, 8759‒8769. |
|
|
|
|
|
|
|
2. M. Šekutor, K. Molčanov, L. Cao, L. Isaacs, R. Glaser, K. Mlinarić-Majerski, Design, Synthesis, and X-ray Structural Analyses of Diamantane Diammonium Salts: Guests for Cucurbit[n]uril (CB[n]) Hosts, Eur. J. Org. Chem. 2014, 2533‒2542. |
|
|
|
|
|
|
|
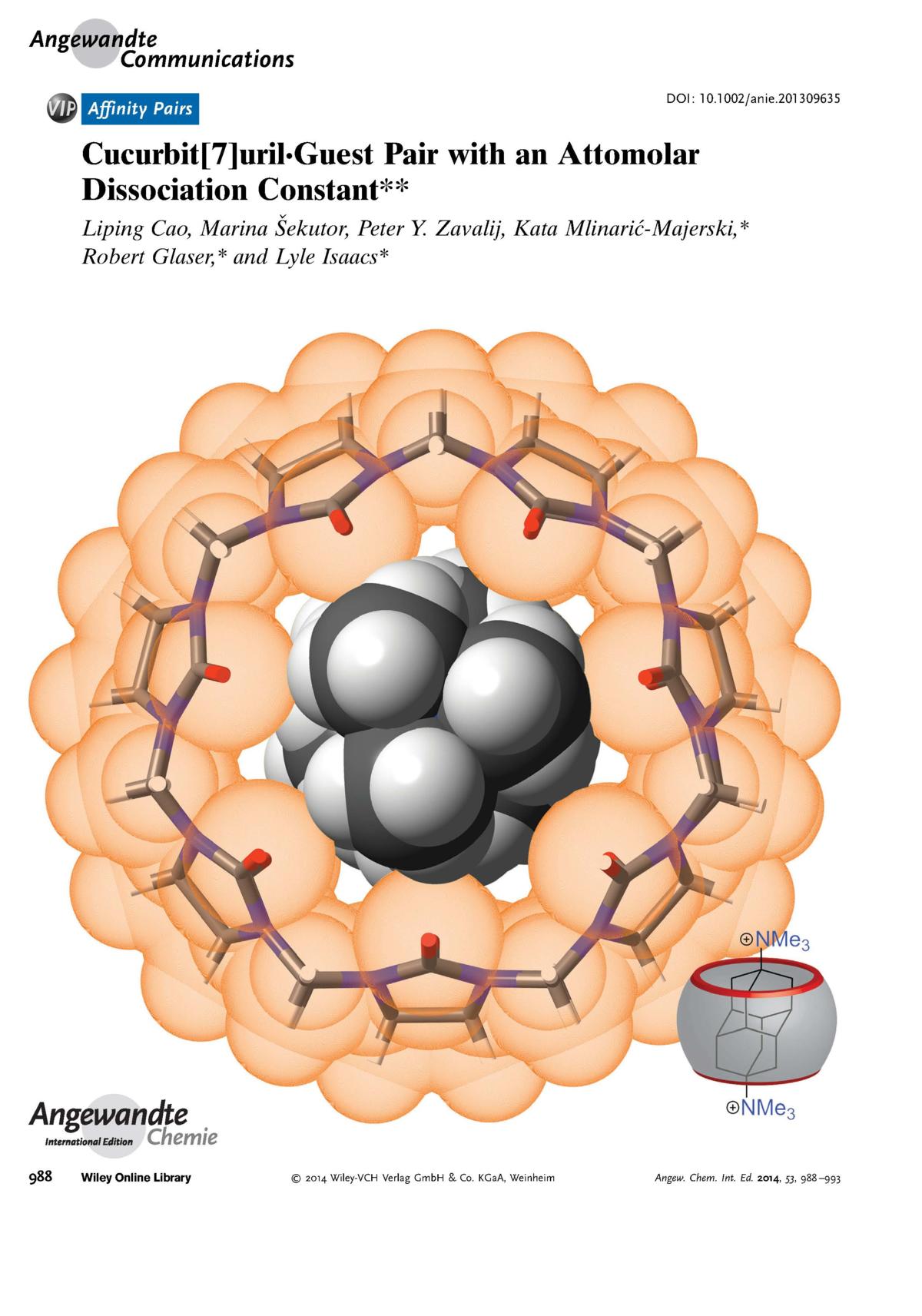
1. L. Cao, M. Šekutor, P. Y. Zavalij, K. Mlinarić-Majerski, R. Glaser, L. Isaacs, Cucurbit[7]uril•Guest Pair with an Attomolar Dissociation Constant, Angew. Chem., Int. Ed. 2014, 53, 988‒993; Angew. Chem. 2014, 126, 1006‒1011. |
|
|
|
|
|
|