TRANS-ZEBRATOX
Principal investigator
Identification and functional characterization of (eco)toxicologically relevant polyspecific membrane transport proteins in zebrafish (Danio rerio)
Introduction
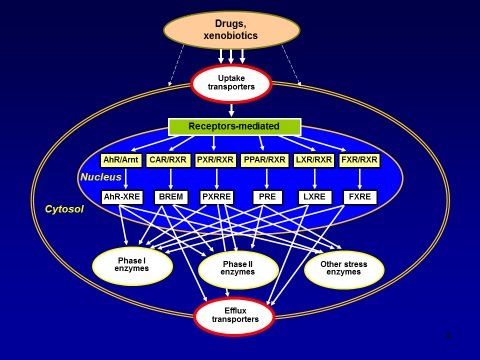
Absorption, distribution, metabolism and excretion (ADME) of chemicals are increasingly recognized as important determinants in the biological activity and/or toxicity of endo- and xenobiotics. A coordinated system of transport proteins, channels, receptors and enzymes act as cellular gatekeepers to foreign molecules, critically determining the so-called ADME-Tox properties of a molecule (1,2). It has recently become evident that interactions with membrane transport proteins are key determinants for crossing tissue barriers, in addition to the basic physical properties of a compound. Specific transmembrane proteins are essential components of this complex cellular defence system (Fig. 1), and the two classes of membrane transporters are particularly relevant: the so-calleduptaketransportersand theefflux transporters.
Whereas most membrane transporters are oligospecific, i.e., specialized for translocation of specific metabolic or nutritional compounds,polyspecific transportersaccept compounds with different sizes and molecular structures. The polyspecific uptake transporters from theSLC (Solute carriers) superfamilyof proteins, the largest of all known protein families, are essential in mediating the entrance of a large number of xenobiotics into the cells (3). After enteringthe cells by transporter mediated uptake, lipophilic xenobiotics are generally biotransformed by phase I and phase II metabolic enzymes into more water-soluble metabolites whose exit from the cells requires efflux transporters. Polyspecific efflux transporters from theABC (ATP Binding Cassette)and theMATE (Multidrug and Toxic compound Extrusion)protein families have been shown to efflux xenobiotics out of cells and serve as critical defence system against the accumulation of xenobiotics and their metabolites in (4-8).
The functional importance of any given transporter in each tissue depends on its substrate specificity, expression level, and the presence/absence of other transporters with overlapping substrate preferences. The regulation of gene expression of various phase I and II metabolising enzymes and membrane transporters has potential impact on the metabolism, elimination, pharmacokinetics/dynamics, toxicokinetics/dynamics and drug-drug interactions of many therapeutic agents, as well as their ability in the protection of the body against the exposure to environmental xenobiotics (2,3). Consequently,in vitrostudies on identification and detailed molecular characterization of membrane transporters, followed or complemented by in vivo evaluation of its physiological and/or defence role in suitable animal research models, represents an important and well established research avenue in pharmacology and toxicology.
From the viewpoint of environmental toxicology, however, the same cellular defence systems are likely to be involved in processing of endo- and xenobiotics in other animals. Aquatic organisms are continuously exposed to a variety of environmental chemicals originating from various anthropogenic sources. Evolutionary conserved cellular defence mechanisms that mediate the overall bioavailability and potential toxicity of xenobiotics are similar to those described in mammals: regulation of absorption by uptake transporters, the well documented biotransformation by the phase I and phase II enzymes, and finally more recently demonstrated active efflux of xenobiotics by the ABC transporters (8). Nevertheless, despite its potential relevance, research directed on the role of membrane transporters in ecotoxicology is truly lagging behind comparative efforts in the mainstream toxicology.
Figure 1. A schematic representation of drugs/chemicals/xenobiotics-induced stress response leading to the activation of specific uptake transporters, receptor-mediated gene expression of phase I and II drug metabolizing enzymes, other stress enzymes, and efflux transporters.
Therefore, continuing the pioneering research our group did on the efflux transporters (9,10), and more recently on the uptake transporters (11,12), in this research proposal we aim to identify and characterize new, potentially (eco)toxicologically relevant uptake and efflux transport proteins not addressed so far in the context of environmental toxicology, nor in non-mammalian species in general. Among the polyspecific uptake transporters, our research will be focused on members of the two SLC families –SLC21 and SLC22– that play important role in cellular uptake of endo- and xenobiotics in mammals. Among the efflux transporters, in this project we plan to focus on more recently discovered efflux proteins that may be of considerable (eco)toxicological relevance: theMATEproteins, and theRLIP76transporter. The proposed research directed to detailed functional characterization, and especially knockout/functional genomics studies, will be focused only on polyspecific transporters that mediate transport of a wide range of xenobiotics, or transporters that may have profound physiological effects, e.g., on bile flow or intestinal absorption, which indirectly affect the absorption, disposition and toxicity of xenobiotics. In order to realize this general goal, we will utilizezebrafishas a well established research model increasingly used both in biomedical and environmental research.
Zebrafish as a Research Model vs. Polyspecific Membrane Transporters
Small fish are invaluable research models for many fields, including biomedicine, cell biology, developmental biology, pharmacology, and environmental toxicology. Zebrafish (Danio rerio) is a unique vertebrate model system that offers numerous advantages not readily offered by other vertebrate models: small size, numerous offspring, short generation time, optical transparency of the embryo, amenability to genetic and chemical screens, cost-benefit ratio that is not possible to match using other vertebrate systems etc. Furthermore, numerous protocols for molecular biological methods, mutagenesis studies, screening strategies and toxicological tests, in addition to functional genomics tools, are established for zebrafish. Also, the availability of thousands of existing mutants and transgenic lines is a vital resource (13,14). As a consequence, the pharmaceutical industry and environmental science are strongly interested in using zebrafish for drug/pollutant screens and the whole animal toxicology studies. However, as the current knowledge on related membrane transporters in zebrafish is limited, the major research objectives of this proposal are set to significantly overcome this deficiency.
Polyspecific UPTAKE Transporters
During recent years it has become increasingly recognized that members of the two SLC families, SLC21 and SLC22, play important roles in cellular uptake of endo- and xenobiotics, including many drugs, in tissues important for pharmacokinetics, such as the liver, kidneys and intestine. SLC21/SLCOsuperfamily (gene name) encompassesorganic anion transporting polypeptides (OATPs), whereasSLC22Asuperfamily includesorganic cation transporters (OCTs),organic carnitine transporters (OCTNs)and theorganic anion transporters (OATs)(15). Many of these proteins are important for the uptake of toxic compounds from the circulation, thus enabling their metabolization and/or subsequent excretion from the body. Nevertheless, despite their physiological importance and well documented role in the cellular detoxification in mammals, none of these transporters had been characterized in non-mammalian organisms before our recent studies on zebrafish (11,12), and the knowledge about polyspecific uptake transporters in non-mammalian species remains truly modest. More specifically:
Organic anion transporting polypeptides (OATPs).OATPs (Oatps in non-human species) are a group of polyspecific transporters that mediate transport of large amphipathic molecules across cell membranes of eukaryotes. OATPs transport a wide range of endogenous (steroid hormones, bile salts, prostaglandins) and exogenous compounds (pharmaceuticals, natural toxins). The classification in mammals includes 6 families named OATP1 6/Oatp1-6. The OATP/Oatp superfamily has been scarcely studied in non-mammalian vertebrates, and a detailed functional analysis of non-mammalian Oatps is lacking. We have previously shown that teleost fish lack Oatp1a and Oatp1b subfamilies but express evolutionary distinct Oatp1d subfamily (11). As teleosts do not have clear orthologs of mammalian OATP1A/Oatp1a and OATP1B/Oatp1b subfamily members, we hypothesized that zebrafish Oatp1d1 has a similar role as OATP1A/Oatp1a and OATP1B/Oatp1b subfamily members in mammals. In order to test this hypothesis we conducted a detailed functional characterization of Oatp1d1 in zebrafish (12). Our results enabled better understanding of the OATP/Oatp evolution within the vertebrate phyla, and offered the first insight into the function and structural properties of a novel zebrafish Oatp1 ortholog. Finally, our most recent work showed that zebrafish Oatp1d1 is involved in the uptake of a considerable diversity of environmentally relevant contaminants into liver, brain and testes, and the inhibition of Oatp1d1 by many environmental contaminants emphasizes their potential role in modulating uptake processes. Therefore, based on our initial data on the tissue specific expression pattern of zebrafish Oatps, and available evidence from mammalian studies, our further research on zebrafish Oatps will be focused on knockout studies onOatp1d1, and functional characterization ofOatp2b1 shown to be highly expressed in the liver, kidney, intestine and gills.
Organic anion transporters (OATs in humans, Oats in other animals). OATs/Oats are a group of polyspecific transporters encoded by theSLC22/Slc22 gene family. They mediate transport of a diverse range of low molecular weight substrates including steroid hormone conjugates, biogenic amines, various drugs and toxins (reviewed in 15-17). They are considered to be a part of an evolutionarily conserved defence system, protecting higher organism from environmental, potentially toxic compounds. Our goal in this project will be to get the first insights into protective role of specific Oats in zebrafish and evaluate their interaction with environmental contaminants. Our preliminary investigations on phylogeny and tissue distribution of Oats in zebrafish already enabled focusing of our studies on selected Oats highly expressed in the liver, kidney, intestine and/or gills. Furthermore, for some of the selected zebrafish Oats (provisionally annotated asOat-like1,Oat1/3,Oat2c andOat2d) we were able to perform the first transient transfection studies in HEK293 cells, as well as the transport activity determinations. Our further research on Oats will include a detailed functional characterization of four selected Oats, followed by knockout studies that will be performed on 1-2 Oats that will show the highest ecotoxicological relevance based on the functional characterization of these proteins.
Organic cation transporters (OCTs in humans, Octs in other animals). OCTs/Octs belong to the SLC22 family that contains three groups of organic cation transporters: (1)OCT1 (SLC22A1), OCT2 (SLC22A2) and OCT3 (SLC22A3), (2) cation and carnitine transporter OCTN1 (SLC22A4), the Na+ carnitine cotransporter OCTN2 (SLC22A5), and (3) cation transporter OCT6 (SLC22A16) (17). Recent substrate specificity studies revealed that OCT1, OCT2 and OCT3 mediate transport of a broad range of organic cations by the means of facilitated diffusion. Substrates ofSLC22 family include a wide variety of structurally diverse small organic cations, both endogenous and exogenous, including many drugs. Similar to the other classes of polyspecific uptake transporters, related studies on non-mammalian species are scarce and there are no studies published on Octs in the environmental context. Our preliminary experiments revealed the presence of a series of polyspecific Octs in zebrafish. Along with the mRNA expression profiling, those data will serve as a starting base for prioritization ofzebrafish Octs that will be subject of detailed characterization within this project.
Polyspecific EFFLUX Transporters
Considering the fact that ABC proteins have been the only type of efflux transporters studied so far in the context of environmental toxicology, in this project, we will focus on different, more recently discovered efflux proteins that may be of considerable (eco)toxicological relevance: the MATEs and the RLIP76 transporters.
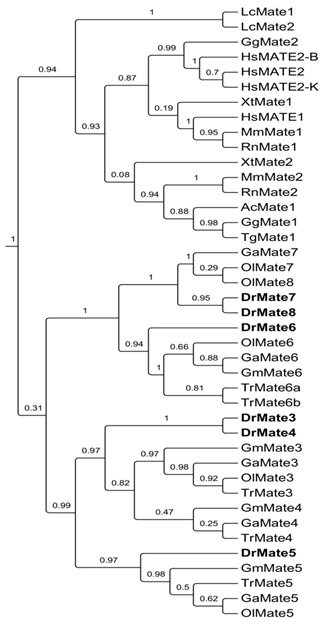
MATE (multidrug and toxic compound extrusion) proteins. MATE/Mate protein family (gene name SLC47) belongs to the SLC superfamily and represents the most recently discovered family of multidrug transporter proteins (6). They are evolutionary conserved from bacteria to mammals, but their function is far from understood. They function as bidirectional transporters where the efflux of substrates is linked with the proton-coupled electroneutral exchange (7). MATEs have only recently been characterized in mammals, and include three proteins in humans (MATE1, MATE2 and MATE2K) and two proteins in rodents (Mate1 and Mate2). MATEs are generally ubiquitously expressed, with the highest expression in kidney and liver. Recently discovered physiological substrates of MATEs include creatinine, thiamine and estrone-3-sulphate, whereas xenobiotic substrates include antiviral drugs, metformin and some model cationic substrates (e.g. cationic dye ASP+). Known inhibitors of MATEs include choline, corticosterone and serotonin as well as xenobiotics cimetidine, nicotine and imatinib. Again, although MATE proteins are recognized as a crucial part of the extrusion mechanism for foreign compounds in mammals, knowledge about ecotoxicological relevance of MATE proteins is lacking. Consequently, one of the project research objectives is to identify and functionally characterize MATE transporters in zebrafish. In order to position and annotate zebrafish MATEs in relation to the other phyla, we already performed initial phylogenetic analyses, and our first data indicate the presence of five MATE genes in zebrafish (provisionally annotated asDrMate3-7), as well as in the other teleost species. The next project steps will be directed to functional characterization of five identified zebrafish MATEs.
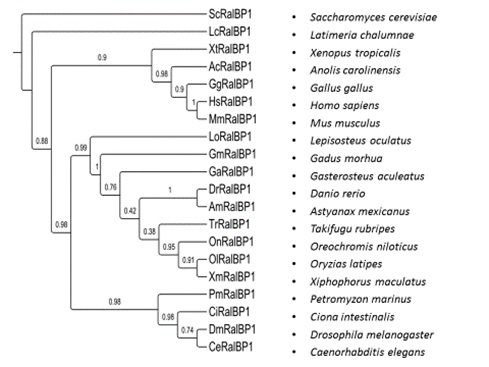
RLIP76 (RalA-binding protein 1). RLIP76 is the most recently discovered stress-responsive, multi-functional membrane protein, found throughout animal kingdom from invertebrates to humans (18). As a stress-responsive membrane protein RLIP76 is involved in the regulation of multiple cellular signalling pathways. It represents the predominant glutathione-conjugate (GS-E) transporter in cells, which emphasize its crucial physiologic function in regulation of intracellular concentration of electrophilic intermediates of oxidative lipid metabolism, by mediating efflux of GS-E, including leukotrienes and the 4-hydroxynonenal-GSH (4HNE-GSH) conjugates. Furthermore, it has been shown that RLIP76 plays a crucial role in defence against radiation and chemotherapeutic toxicity, by preventing the apoptosis of cancer cells (19). RLIP76 substrates range from weakly cationic compounds to anionic metabolites like glutathione conjugates of electrophiles. Any knowledge on Rlip76 characteristics and functions in zebrafish and in non-mammalian species in general, is lacking. Our preliminary qRT-PCR analysis revealed tissue expression pattern of Rlip76 in zebrafish. Putting these initial data into a context, together with reported broad substrate affinities of RLIP76, over-expression in cancer tissues, and finally ubiquitous expression in different tissues, we believe that Rlip76 may be an important cellular defence factor that contributes to the excretion of endobiotic and xenobiotic metabolites in fish. Therefore, using similar methodological framework as described in the case of other polyspecific transporters, in this project, we plan to perform a detailed functional characterization ofzebrafish Rlip76 as a newly identified efflux transporter of potential (eco)toxicological relevance. In addition, we will try to develop the Rlip76 knock out and GFP reporter system forin vivo promoter analysis in order to better understand its transcriptional regulation. These zebrafish models could potentially provide insights into the underlining molecular mechanisms of dual role of Rlip76 in metabolism and cancer.
Main Objectives and Potential Relevance
As explained and justified above, the main goal of the proposed project is identification and functional characterization of (eco)toxicologically relevant polyspecific uptake and efflux transporters in zebrafish as an important research model. The main goal will be reached through implementation of the following main objectives:
1) Identification of genes coding for polyspecific uptake and efflux transport proteins in zebrafish genome, and determination of their phylogenetic relationships with other taxa;
2) Selection of the genes of potential (eco)toxicological relevance that will be addressed in more detail in the next project steps; tu bi spojila filo+ekspresija za seleciju gena!
3) Functional/molecular characterization of selected uptake and efflux transporters, by cloning, transfection, transport activity assays, and determinations of evolutionary conserved structural properties, using appropriate heterologous expression systems;
4) Development and standardization of the high-throughput-screening (HTS) methods and identification of interactors of target transport proteins among environmentally relevant contaminants;
5) In vivoevaluation of physiological and/or defence function, and potential environmental relevance of the target transporter(s), using the zebrafish functional genomics tools.
Generation of new knowledge from this type of project is a necessary prerequisite for better understanding of the ADME-Tox properties of drugs and/or environmental contaminants. This will in turn improve our understanding and predictions of toxicity, and enable a more reliable risk assessment in the context of both human and environmental toxicology. In addition, apart from new insights relevant for our fundamental understanding of the evolution, biological role and (eco)toxicological significance of polyspecific membrane transporters, accomplishment of the proposed research plan will result in standardization of new methods with the high-throughput-screening (HTS) potential. Such methods can be readily developed in order to allow reliable, rapid and low-cost evaluation of interactions of existing or new drugs and/or environmental contaminants with specific transport proteins. Finally, considering there is no zebrafish facility available in Croatia at this point, we believe that the proposed initial setup of a small scale zebrafish facility, with a competitive set of the zebrafish functional genomics tools, will represent a new and highly valuable national and regional resource for many researchers working in the fields of biology, biomedicine and environmental science.
Methodology and Resources
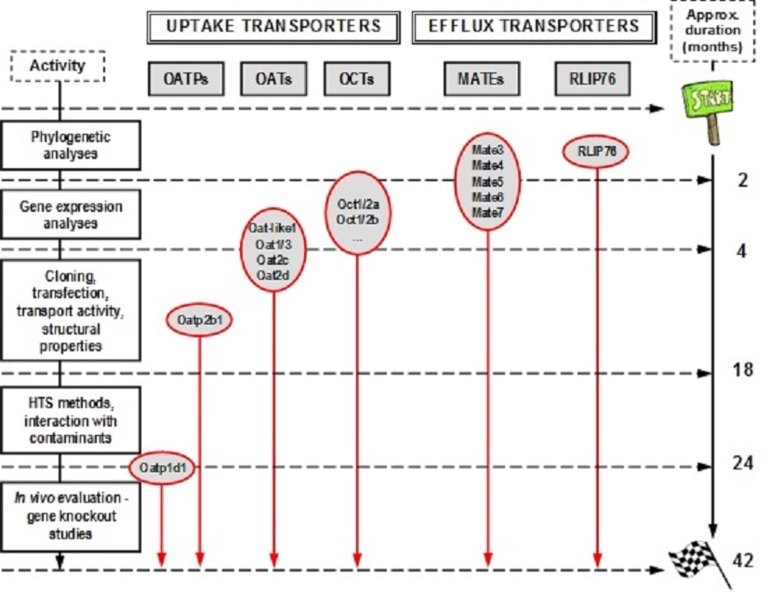
A general methodological concept of the work planned is schematically presented in Fig. 1, with indications of critical time-points in relation to the present state of our research on target classes of transporters, and expected progress related to the proposed research plan. In addition to the detailed methodological approach described inour recent studies (11,12), in this project we plan to add a novel research step focused on the in vivo evaluation of the (eco)toxicological relevance of a few carefully selected transporters that will be characterized in detail in previous project phases. This final step will be accomplished using the zebrafish functional genomics tools based on the gene targeting/knockout studies, utilizing customized transcription activator-like effector nucleases (TALENs) approach (20), followed by the analyses of the relevant phenotype changes in embryos and adults (21).
Figure 2. Methodological scheme of the project, with indications of the critical time-points in relation to the present state of our research on target classes of transporters, and the proposed research plan.
The proposed duration of the project is3.5 years(42 months). Costs specified in the Financial plan include realistic projections of expenses, adjusted to the type, expected duration and intensity of specific project steps. The project team consists of the PI (Dr. Tvrtko Smital), 1 research associate (Dr. Roko Zaja), 4 PhD students/postdocs (Marta Popovic, Ivan Mihaljevic, Petra Maric, Jelena Dragojevic), and 1 research assistant (BSc Jovica Loncar). Apart from the full-scale equipment for the zebrafish functional genomics, the project team has expertise and equipment needed for successful accomplishment of the declared project objectives.
Selected Project Results
Prikaz odabranih rezultata vezanih uz cilj 1. – Identifikacija gena kodirajućih za polispecifične uptake i efflux transportne proteine u genomu zebrice (SLC22 i MATE (SLC47A) porodice te RLIP76) i filogenetska usporedba s drugim vrstama.
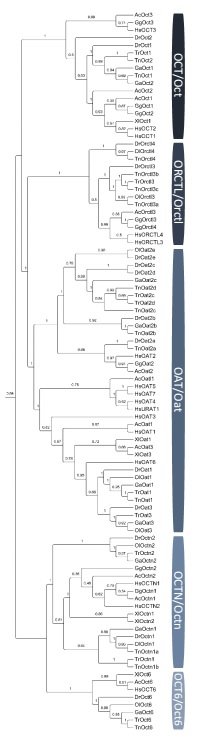
Slika 3. Filogenetsko stablo SLC22 porodice transportnih proteina u kralješnjaka. Kratice vrsta: Hs,Homo sapiens; Gg,Gallus gallus; Ac,Anolis carolinensis; Xl,Xenopus laevis; Dr,Danio rerio; Ga,Gastrosteus aculeatus; Ol,Oryzes latipes; Tn,Tetraodon nigroviridis; Tr,Takifugu rubripes.
(Mihaljević i sur., znanstveni rad u pripremi)
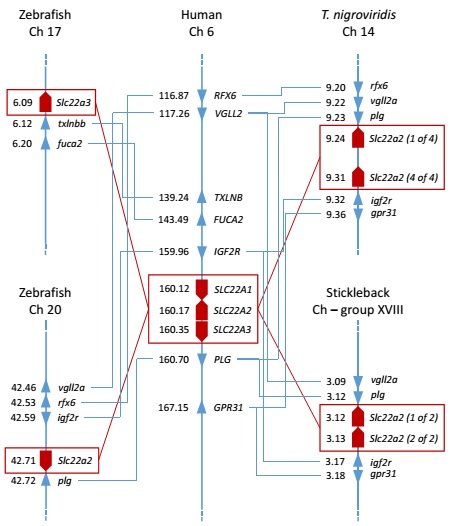
Slika 4. Analiza sintenije između OCT/Oct gena zebrice, čovjeka i odabranih ribljih vrsta. Brojevi uz imena gena predstavljaju parove megabaza (megabase pair – Mbp) na odgovarajućoj lokaciji gena na kromosomu.
(Mihaljević i sur., znanstveni rad u pripremi)
Slika 5. Filogenetsko stablo MATE (SLC47A) porodice transportnih proteina u kralješnjaka. Kratice vrsta: Hs,Homo sapiens; Mm –Mus musculus;Gg,Gallus gallus; Ac,Anolis carolinensis; Xl,Xenopus laevis; Dr,Danio rerio; Ga,Gastrosteus aculeatus; Ol,Oryzes latipes; Tn,Tetraodon nigroviridis; Tr,Takifugu rubripes.
(Popović i sur., znanstveni rad u pripremi)
Slika 6. Filogenetsko stablo RLIP76 (RALBP) transportnog proteina, temeljeno na dostupnim genskim sekvencama vrsta iz različitih taksonomskih kategorija.
Prikaz odabranih rezultata vezanih uz cilj 2. – Odabir gena od potencijalne (eko)toksikološke važnosti u svrhu detaljne karekterizacije u narednim fazama projekta.
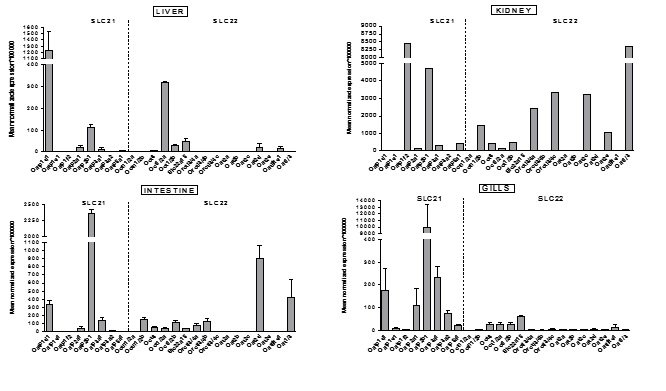
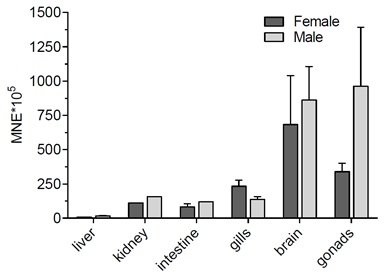
Slika 7. Opći pregled ekspresije gena kodirajućih za transportne proteine iz SLC21 i SLC 22 porodice u odabranim tkivima odraslih zebrica ženskog spola. Podaci predstavljaju srednju normaliziranu ekspresiju (mean normalized expression – MNE) ± SD normaliziranu na kontrolni (housekeeping) gen Ef1α.
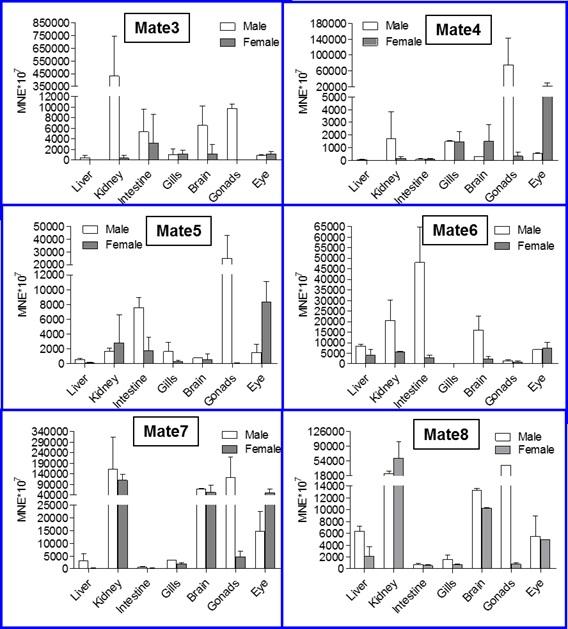
Slika 8. qRT-PCR analiza ekspresije MATE (SLC47A) gena u tkivima odraslih zebrica oba spola. Podaci predstavljaju srednju normaliziranu ekspresiju (mean normalized expression – MNE) ± SD normaliziranu na kontrolni (housekeeping) gen Ef1α.
(Popović i sur., znanstveni rad u pripremi)
Slika 9. qRT-PCR analiza ekspresije RLIP76 (RALBP) gena u tkivima odraslih zebrica oba spola. Podaci predstavljaju srednju normaliziranu ekspresiju (mean normalized expression – MNE) ± SD normaliziranu na kontrolni (housekeeping) gen Ef1α.
Prikaz odabranih rezultata vezanih uz cilj 3. – Funkcionalna/molekularna karakterizacija odabranih uptake i efflux transportnih proteina.
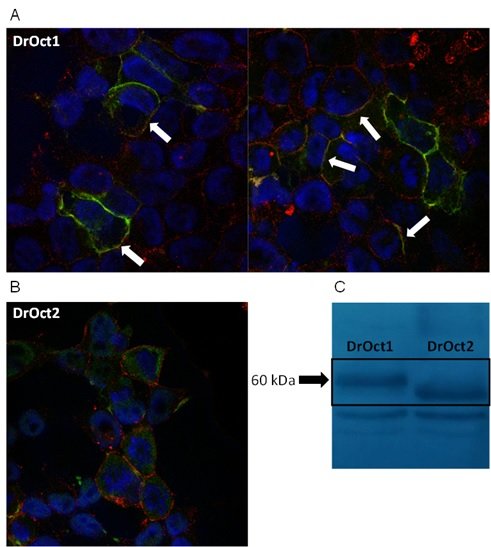
Slika 10. Stanična lokalizacija Oct1 (A) i Oct2 (B) transportnih proteina zebrice u HepG2 stanicama prolazno transfeciranim ciljanim genima/proteinima označenim N-terminal Xpress biljegom. C: Western blotting ciljanih Oct1 i Oct2 proteina zebrice.
(Mihaljević i sur., znanstveni rad u pripremi)
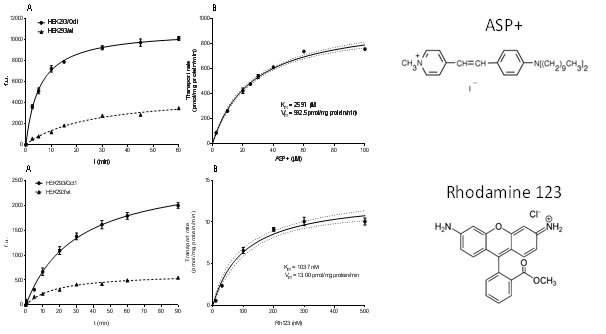
Slika 11. Michaelis-Menten kinetika unosa modelnih fluorescentnih supstrata ASP+, odnosno rodamin 123 (Rh123), putem Oct1 transportnog proteina zebrice. A) Vremenski ovisni unos fluorescentnih supstrata u HEK293 stanice s prekomjernom ekspresijom Oct1, odnosno u HEK293 stanice divljeg tipa (stanice netransfecirane Oct1 genom zebrice). Akumulacija modelnih supstrata određivana je kao uvećanje fluorescencije (fluorescentne jedinice – f.u.) u vremenu (min). B) Doza-odgovor krivulja ASP+, odnosno Rh123 unosa putem Oct1 izražena kao rata transporta (pmol/mg proteina/min) u odnosu na koncentraciju ASP+, odnosno Rh123 (µM), nakon 5 min inkubacije stanica s modelnim supstratima.
(Mihaljević i sur., znanstveni rad u pripremi)
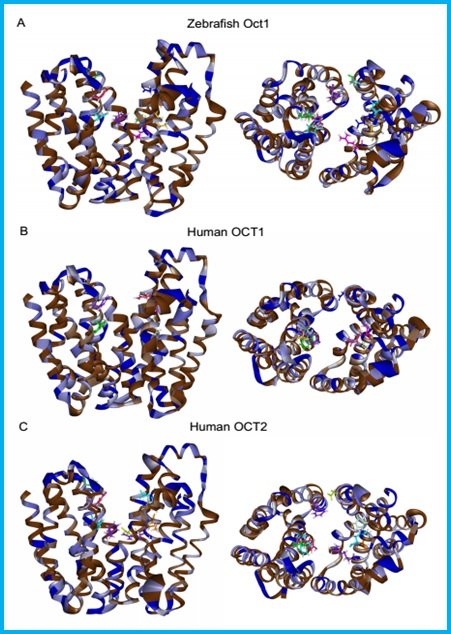
Slika 12. Homologno molekularno modeliranje i smještanje (docking) Oct1 transportnog proteina zebrice u odnosu na humani OCT1 i OCT2 – neizravna potvrda kompleksnosti i prostornosti aktivne regije Oct1 proteina zebrice.
(Mihaljević i sur., znanstveni rad u pripremi)
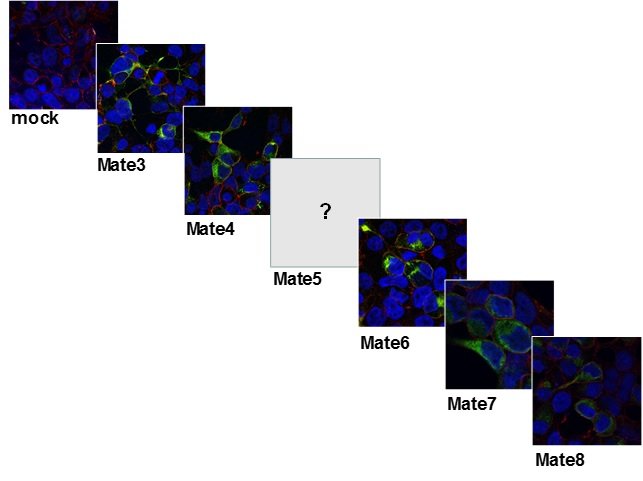
Slika 13. Stanična lokalizacija MATE (DrMate3, 4, 6-8) transportnih proteina zebrice u HepG2 stanicama prolazno transfeciranim ciljanim genima/proteinima označenim GFP biljegom.Napomena: u trenutku pisanja Izvješća još uvijek nismo uspjeli zadovoljavajuće klonirati i eksprimirati DrMate5.
(Popović i sur., znanstveni rad u pripremi)
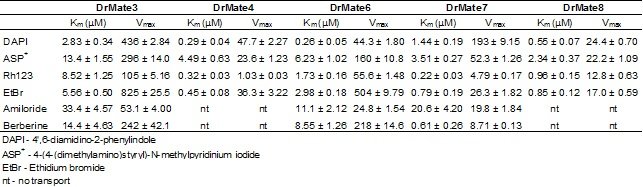
Tablica 1. Temeljni kinetički parametri, Km (µM) i Vmax (pmol/mg protein/min) unosa/transporta fluorescentnih modelnih supstrata (4-(4-(dimetilamino)stiril)-N-metilpiridinij jodid (ASP+); 4',6-diamidino-2-fenilindol (DAPI); rodamin 123 (Rh123); etidij bromid (EtBr), berberin i amilorid) posredstvom MATE proteina zebrice: DrMate3, DrMate4, DrMate6, DrMate7 i DrMate8. Vrijednosti predstavljaju srednje vrijednsoti + SD mjerenja u triplikatu.
(Popović i sur., znanstveni rad u pripremi)
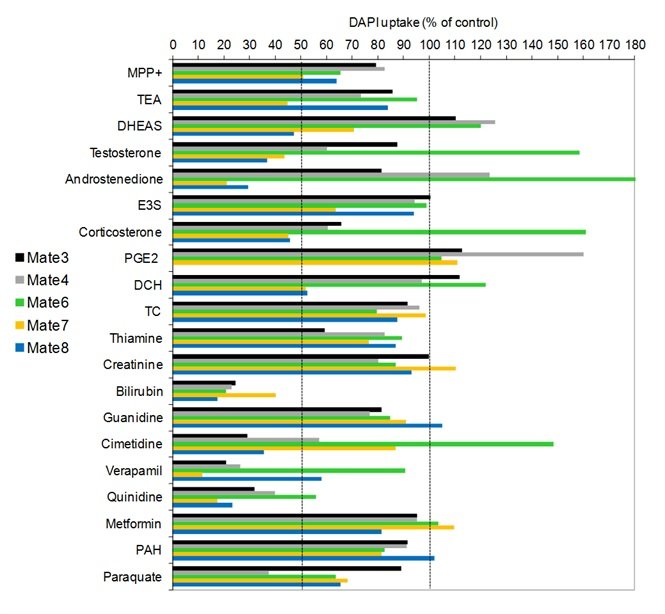
Slika 14. Interakcija MATE proteina (DrMate3, 4, 6, 7 i 8) zebrice s odabranim fiziološkim i okolišno relevantnim spojevima. Interakcija je određivana metodom mjerenja unosa modelnog fluorescentnog supstrata 4',6-diamidino-2-fenilindola (DAPI) u HepG2 stanice prolazno transfecirane genima kodirajućim za testirane MATE proteine zebrice.
(Popović i sur., znanstveni rad u pripremi)
Kupnja i instalacija opreme za mikroinjektiranje ribljih embrija – cilj 7.
Slika 15. Sustav za mikroinjektiranje embrija zebrice: stereo mikroskop SMZ-171-TLED proizvođača Motic opremljen 5 MP digitalnom kamerom Moticam 5, te mikroinjektor FemtoJet 4x proizvođača Eppendorf. Sustav je instaliran u Laboratoriju za molekularnu ekotoksikologiju, Zavoda za istraživanje mora i okoliša Instituta Ruđer Bošković.
Key References
(1) Ekins S, Nikolsky Y, Nikolskaya T (2005) Trends Pharmacol Sci 26: 202-209; (2) Xu C, Yong-Tao L, Kong T (2005) Arch Pharm Res 28: 249-268; (3) Klaassen CD, Lu H (2008) Toxicol Sci 101: 186-196; (4) Sarkadi B, Homolya L, Szakacs G, Varadi A (2006) Physiol Rev 86: 1179-1236; (5) Szakács G, Váradi A, Özvegy-Laczka C, Sarkadi B (2008) Drug Discov Today 13: 379–393; (6) Damme K, Nies AT, Schaeffeler E, Schwab M (2011) Drug Metabol Rev 43: 499-523; (7) Klaassen CD, Aleksunes LM (2010) Pharmacol Rev 62: 1–96; (8) Epel D, Stevenson CN, MacManus-Spencer LA, Luckenbach T, Hamdoun A, Smital T (2008) Environ Sci Tech 42: 3914-3920; (9) Kurelec B (1992) Crit Rev Toxicol 22: 23-43; (10) Smital T, Luckenbach T, Sauerborn R, Hamdoun AM, Vega RL, Epel D (2004) Mut Res 552: 101-117; (11) Popović M, Žaja R, Smital T (2010) Comp Biochem Physiol A 155: 327-335; (12) Popovic M, Zaja R, Fent K, Smital T (2013) J Biol Chem (accepted for publication/in press); (13) Tiefenbach J, Moll PR, Nelson MR, Hu C, Baev L, Kislinger T, Krause HM (2010) PLoS ONE 5: e9797 doi:10.1371/journal.pone.0009797; (14) Huang P, Xiao A, Zhou M, Zhu Z, Lin S, Zhang B (2011) Nat Biotechnol 29: 699-700; (15) Roth M, Obaidat A, Hagenbuch B (2012) Brit J Pharmacol 165: 1260–1287; (16) Burckhardt G (2012) Pharmacol Ther 136: 106-130; (17) Koepsell H (2013) Mol Aspects Med 34: 413-435; (18) Yadav S, Zajac E, Singhal SS, Awasthi S (2007) Cancer metastasis reviews 26: 59–69; (19) Singhal SS, Sehrawat A, Sahu M, Singhal P, Vatsyayan R, Lelsani PCR, Yadav S, Awasthi S, (2010) International journal of cancer 126: 1327–1338; (20) Moore FE, Reyon D, Sander JD, Martinez SA, Blackburn JS, Khayter C, Ramirez CL, Keith Joung J, Langenau DM (2012) PLoS ONE 7(5): e37877; (21) Taylor KL, Grant NJ, Temperley ND, Patton EE (2010) Cell Commun Signal 8: doi: 10.1186/1478-811X-8-11
Publications