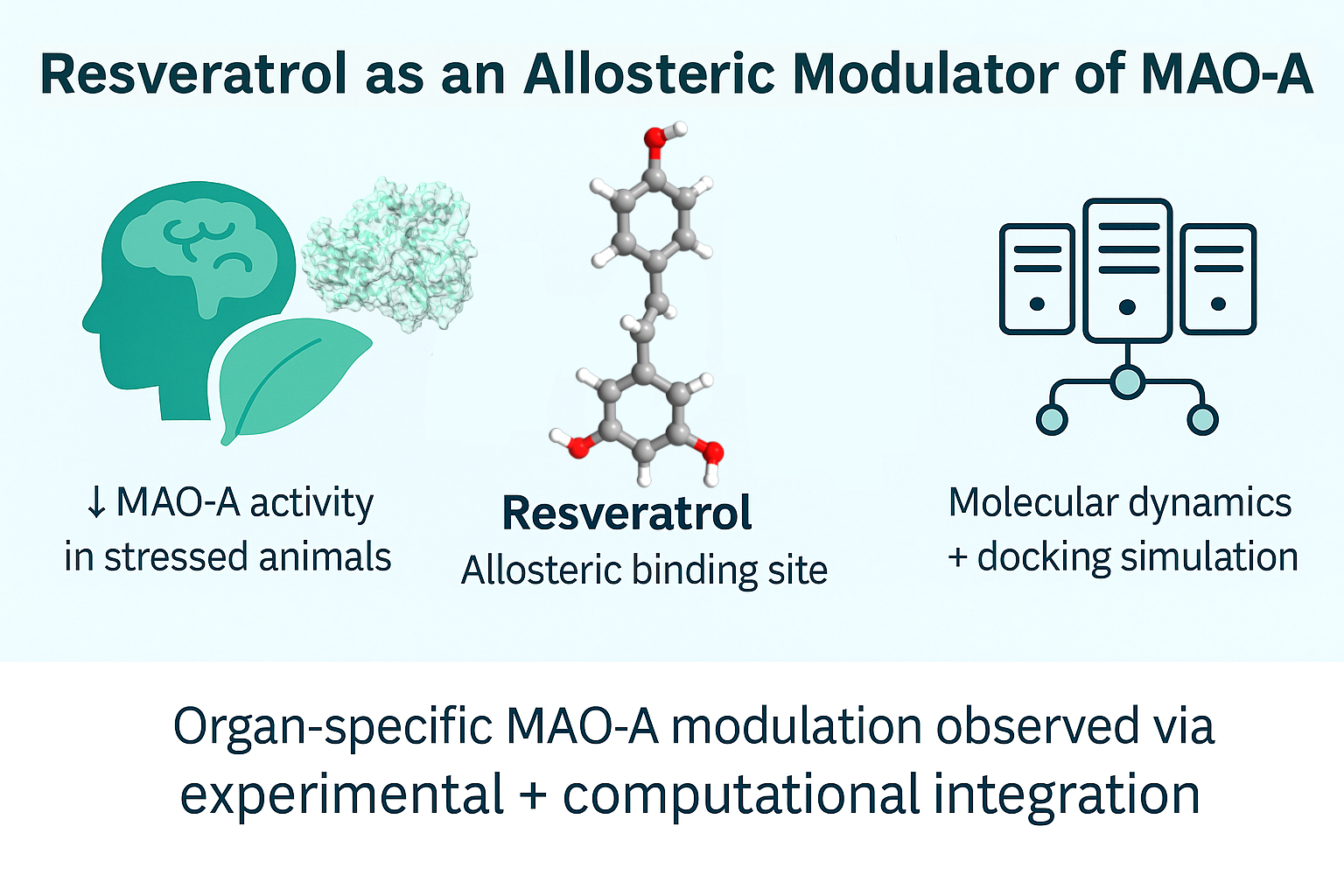
Can Resveratrol Calm the Stressed Brain? HPC and Molecular Modeling Unveil an Allosteric Mechanism of MAO-A Regulation
A recent study published in Biomedicines offers compelling insights into how resveratrol, a natural polyphenol, and its primary metabolite trans-resveratrol-3-O-glucuronide, may serve as allosteric modulators of monoamine oxidase A (MAO-A)—a key enzyme involved in regulating mood-related neurotransmitters. What sets this work apart is its combined use of high-performance computing (HPC), molecular modeling, and robust in vivo experimentation to uncover how chronic stress alters biochemical pathways in the liver–brain axis.
Resveratrol: More Than an Antioxidant
While resveratrol is well-known for its antioxidant and anti-inflammatory properties, this study explores a novel role: direct biochemical modulation of MAO-A under conditions of chronic predator stress in rats. This model closely mimics the physiological impact of prolonged psychological stress, a key factor in anxiety and mood disorders.
Using molecular docking and molecular dynamics simulations powered by HPC resources, the research team discovered that both resveratrol and its metabolite bind to an allosteric site on the MAO-A enzyme—distinct from the catalytic pocket. These interactions were predicted to alter the enzyme’s conformation and reduce its activity.
Experimental Highlights: Biology Meets Simulation
To validate these findings, the team conducted a series of in vivo and biochemical assays:
-
Behavioral Testing: Rats exposed to chronic predator stress underwent elevated plus maze and open field tests, which confirmed increased anxiety-like behavior.
-
Monoamine Oxidase Activity Measurement: MAO-A activity was not only assessed in whole-brain homogenates but also regionally, across the amygdala, hippocampus, and prefrontal cortex—key brain areas involved in emotion regulation. Importantly, the study extended beyond the brain to include hepatic MAO-A, spotlighting the often-overlooked liver–brain axis in stress response.
-
Compound Quantification: Plasma concentrations of resveratrol and trans-resveratrol-3-O-glucuronide were determined using sensitive analytical techniques to correlate systemic exposure with biological outcomes.
-
Results: High-dose resveratrol significantly suppressed MAO-A activity in both brain and liver tissues, particularly in stressed animals—supporting the allosteric inhibition mechanism suggested by simulations.
Role of HPC and Molecular Modeling
This study demonstrates how HPC infrastructure is pivotal in simulating protein-ligand interactions over time, capturing transient conformational changes, water-mediated bonding, and ligand flexibility—all of which are essential for identifying non-classical binding sites like the allosteric pocket found on MAO-A. These molecular insights were then validated by experimental data, showcasing the synergy between computational and biological science.
Broader Implications
By integrating molecular modeling, behavioral neuroscience, and biochemistry, the study not only elucidates a novel mechanism of MAO-A regulation but also proposes a non-invasive, natural compound-based approach to modulate neurotransmitter balance. This opens exciting possibilities for stress-related mood disorder therapies that go beyond traditional MAO inhibitors, which often come with significant side effects.
Conclusion
This work stands as a model for systems-level biomedical research, combining computational power and wet-lab precision to explain how natural compounds can influence complex enzymatic networks under physiological stress. It underscores the growing role of HPC and molecular modeling in guiding both fundamental science and translational research toward next-generation therapeutic strategies.
Read the full paper: https://www.mdpi.com/2227-9059/13/5/1196
Title: Resveratrol and Its Metabolite as Potential Allosteric Regulators of Monoamine Oxidase A Activity in the Brain and Liver Under Chronic Predator Stress
This research was supported by the Russian Science Foundation and the Chelyabinsk Region (project #23-15-20040), the Croatian Science Foundation (grant IP-2022-4658), and the University of Zagreb Computing Center – SRCE, for access to the Supek supercomputer.