Laboratory for Synthetic Organic Chemistry is continuing the tradition of the former Laboratory of Synthetic and Physical Organic Chemistry dealing with the chemistry of cage molecules. Research interests have recently been shifted to three main themes:
- photochemical reaction mechanisms, supramolecular control of photochemical reactivity and development of photocages
- synthesis of host and guest molecules for different supramolecular systems and applications
- synthesis of biologically active molecules
- investigation of diamondoid compounds
Covering various synthetic, analytical and spectroscopic techniques on substrates including polycyclic molecules, heterocycles, and peptides, the research activities in the laboratory are ideal for training young researchers in graduate and post-graduate education (BSc, MSc, and PhD thesis).
- Photochemical reaction mechanisms and supramolecular control of photochemical reactivity
Photochemical reactivity in LSOC is studied on several systems with particular interest in excited state proton transfer (ESPT), different photoelimination reactions and photochemical transformations of N-heterocycles.
The research was funded:
Currently, the research is funded by the Croatian Science Foundation project:
HRZZ-IP-2024-05-8565 Functional Photoreactive Chromophores for Applications in Organic Synthesis and Biology (FunPhotoAppSynBio)
The project implementation is organized on three working packages concerned with three classes of chromophores:
1) New BODIPY derivatives as potential photodrugs, photoswitches and photocages for applications in organic synthesis and biology
2) New carbazole derivatives as precursors of aza-quinone methides, potential photodrugs and precursors in complex organic synthesis
3) New biphenylamine photocages excitable by two-photon absorption with applications in organic synthesis and biology
- Synthesis of host and guest molecules for different supramolecular systems and applications
LSOC has a long tradition in the synthesis and characterization of different supramolecular systems. Interests include:
a) Synthesis of crown ethers and cryptands for the complexation of cations
Synthesis of crown ethers and cryptands with incorporated cage molecules has been conducted in LSOC over past twenty years. Recently, the research is conducted in collaboration with the Marijeta Kralj's group at the RBI with the aim of developing new antiproliferative molecules that target cancer stem cells and base their action on misregulation of potassium.
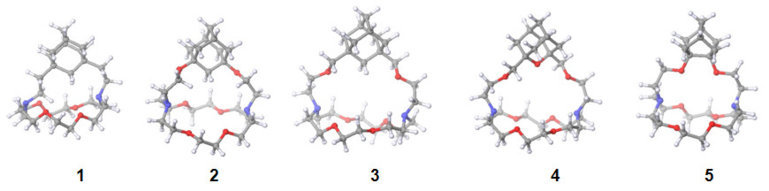
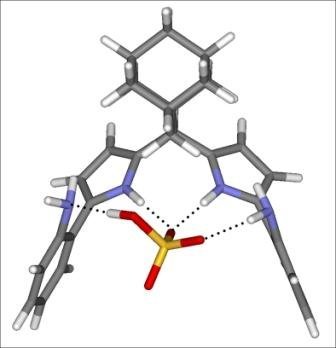
b) Synthesis of anion sensors
Over the past ten years, the synthetic endeavors in the LSOC were oriented to the preparation of fluorescent anion sensors where the recognition of anionic species was accomplished by pyrroles or urea functional groups.
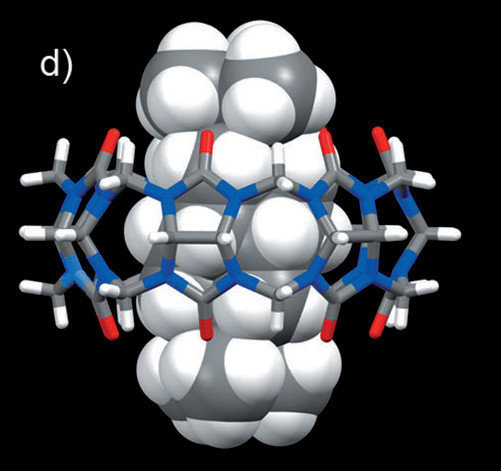
c) Synthesis of diamondoid molecules for the supramolecular systems characterized by high association constants
The research is conducted in collaboration with the group of L. Isaacs (University of Maryland, U.S.A.) and R. Glaser (Ben-Gurion University of the Negev, Israel). LSOC team members designed and prepared various new cage molecules and polycycles - guests for the complexation with cucurbit[n]urils.
- Synthesis of biologically active molecules
Over the years LSOC members have gained significant expertise in preparing different classes of molecules which include: polycycles, cage molecules, heterocycles and peptides. A number of these molecules were screened for their antiproliferative, antiviral or animicrobic activity. Moreover one of the goals in the on-going and the previous HrZZ project is development of phototerapeutics that base their action on photochemical generation of quinone methides. The research is conducted in collaboration with the Marijeta Kralj's group at the RBI.